Endocrinol Metab.
2016 Jun;31(2):206-212. 10.3803/EnM.2016.31.2.206.
Dissecting Tumor-Stromal Interactions in Breast Cancer Bone Metastasis
- Affiliations
-
- 1Department of Molecular Biology, Princeton University, Princeton, NJ, USA. ykang@princeton.edu
- KMID: 2308849
- DOI: http://doi.org/10.3803/EnM.2016.31.2.206
Abstract
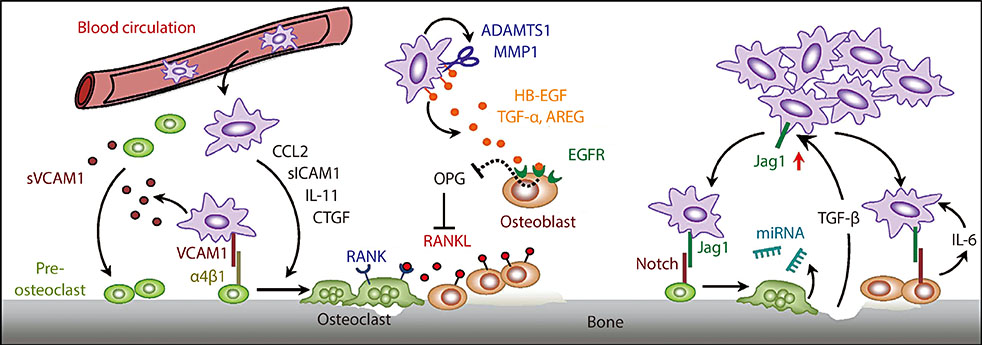
- Bone metastasis is a frequent occurrence in breast cancer, affecting more than 70% of late stage cancer patients with severe complications such as fracture, bone pain, and hypercalcemia. The pathogenesis of osteolytic bone metastasis depends on cross-communications between tumor cells and various stromal cells residing in the bone microenvironment. Several growth factor signaling pathways, secreted micro RNAs (miRNAs) and exosomes are functional mediators of tumor-stromal interactions in bone metastasis. We developed a functional genomic approach to systemically identified molecular pathways utilized by breast cancer cells to engage the bone stroma in order to generate osteolytic bone metastasis. We showed that elevated expression of vascular cell adhesion molecule 1 (VCAM1) in disseminated breast tumor cells mediates the recruitment of pre-osteoclasts and promotes their differentiation to mature osteoclasts during the bone metastasis formation. Transforming growth factor β (TGF-β) is released from bone matrix upon bone destruction, and signals to breast cancer to further enhance their malignancy in developing bone metastasis. We furthered identified Jagged1 as a TGF-β target genes in tumor cells that engaged bone stromal cells through the activation of Notch signaling to provide a positive feedback to promote tumor growth and to activate osteoclast differentiation. Substantially change in miRNA expression was observed in osteoclasts during their differentiation and maturation, which can be exploited as circulating biomarkers of emerging bone metastasis and therapeutic targets for the treatment of bone metastasis. Further research in this direction may lead to improved diagnosis and treatment strategies for bone metastasis.
Keyword
MeSH Terms
-
Biomarkers
Bone Matrix
Breast Neoplasms*
Breast*
Diagnosis
Exosomes
Fractures, Bone
Humans
Hypercalcemia
MicroRNAs
Neoplasm Metastasis*
Osteoblasts
Osteoclasts
Stromal Cells
Transforming Growth Factor beta
Transforming Growth Factors
Vascular Cell Adhesion Molecule-1
Biomarkers
MicroRNAs
Transforming Growth Factor beta
Transforming Growth Factors
Vascular Cell Adhesion Molecule-1
Figure
Cited by 1 articles
-
Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
Hyo Min Jeong, Sun Wook Cho, Serk In Park
Endocrinol Metab. 2016;31(4):485-492. doi: 10.3803/EnM.2016.31.4.485.
Reference
-
1. Guise TA, Kozlow WM, Heras-Herzig A, Padalecki SS, Yin JJ, Chirgwin JM. Molecular mechanisms of breast cancer metastases to bone. Clin Breast Cancer. 2005; 5:Suppl. S46–S53.2. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002; 2:584–593.3. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009; 9:274–284.4. Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011; 11:411–425.5. Kennedy MJ. Metastatic breast cancer. Curr Opin Oncol. 1996; 8:485–490.6. Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009; 27:1564–1571.7. Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011; 377:813–822.8. Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body JJ, et al. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res. 2008; 14:6387–6395.9. Mackiewicz-Wysocka M, Pankowska M, Wysocki PJ. Progress in the treatment of bone metastases in cancer patients. Expert Opin Investig Drugs. 2012; 21:785–795.10. Shen Y, Nilsson SK. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012; 19:250–255.11. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013; 31:285–316.12. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505:327–334.13. Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466:829–834.14. Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495:231–235.15. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481:457–462.16. Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013; 495:227–230.17. Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003; 21:763–770.18. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410:701–705.19. Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011; 12:49–60.20. Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011; 121:1298–1312.21. Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the 'pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev. 2006; 25:521–529.22. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010; 222:268–277.23. Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008; 322:1861–1865.24. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997; 276:71–74.25. Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014; 3:481.26. Sethi N, Kang Y. Dysregulation of developmental pathways in bone metastasis. Bone. 2011; 48:16–22.27. Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015; 27:193–210.28. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.29. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649.30. Clezardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011; 13:207.31. Monteiro AC, Leal AC, Goncalves-Silva T, Mercadante AC, Kestelman F, Chaves SB, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013; 8:e68171.32. Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012; 7:e46342.33. Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012; 189:4258–4265.34. Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003; 3:537–549.35. Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell. 2011; 20:701–714.36. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001; 410:50–56.37. Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009; 284:29087–29096.38. Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009; 23:1882–1894.39. Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011; 19:192–205.40. Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med. 2009; 15:960–966.41. Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013; 24:542–556.42. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Coleman R, Powles T, Paterson A, Gnant M, Anderson S, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015; 386:1353–1361.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
- Advances in Clinically Relevant Metastatic Breast Cancer Models
- Expression of Osteoprotegerin and RANK Ligand in Breast Cancer Bone Metastasis
- Correlation of tumor angiogenesis and lymph node metastasis in invasive breast carcinoma
- Bone-modifying agents for bone metastasis in patients with breast cancer