Intest Res.
2023 Jul;21(3):353-362. 10.5217/ir.2023.00013.
Infectious complications in patients with inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Division of Gastroenterology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Division of Gastroenterology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 4Department of Gastroenterology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 5Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore
- 7Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, India
- KMID: 2544758
- DOI: http://doi.org/10.5217/ir.2023.00013
Abstract
- Background/Aims
Infectious complications are major concerns when treating patients with inflammatory bowel disease (IBD). This study evaluated clinical differences across countries/regions in the management of infectious diseases in patients with IBD.
Methods
A multinational online questionnaire survey was administered to participants at the 8th meeting of the Asian Organization for Crohn’s and Colitis. The questionnaire included questions regarding surveillance, diagnosis, management, and prevention of infection in patients with IBD.
Results
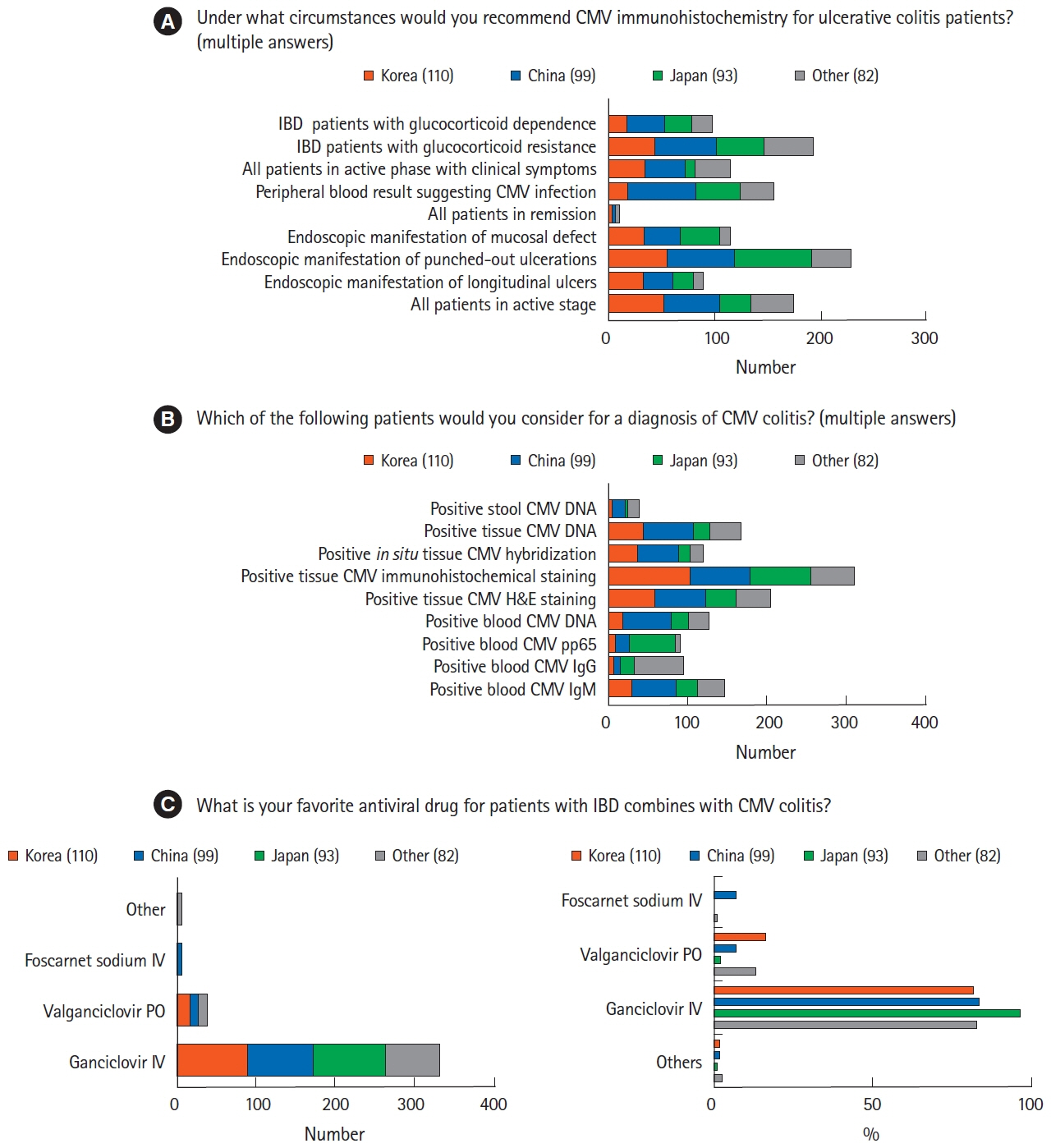
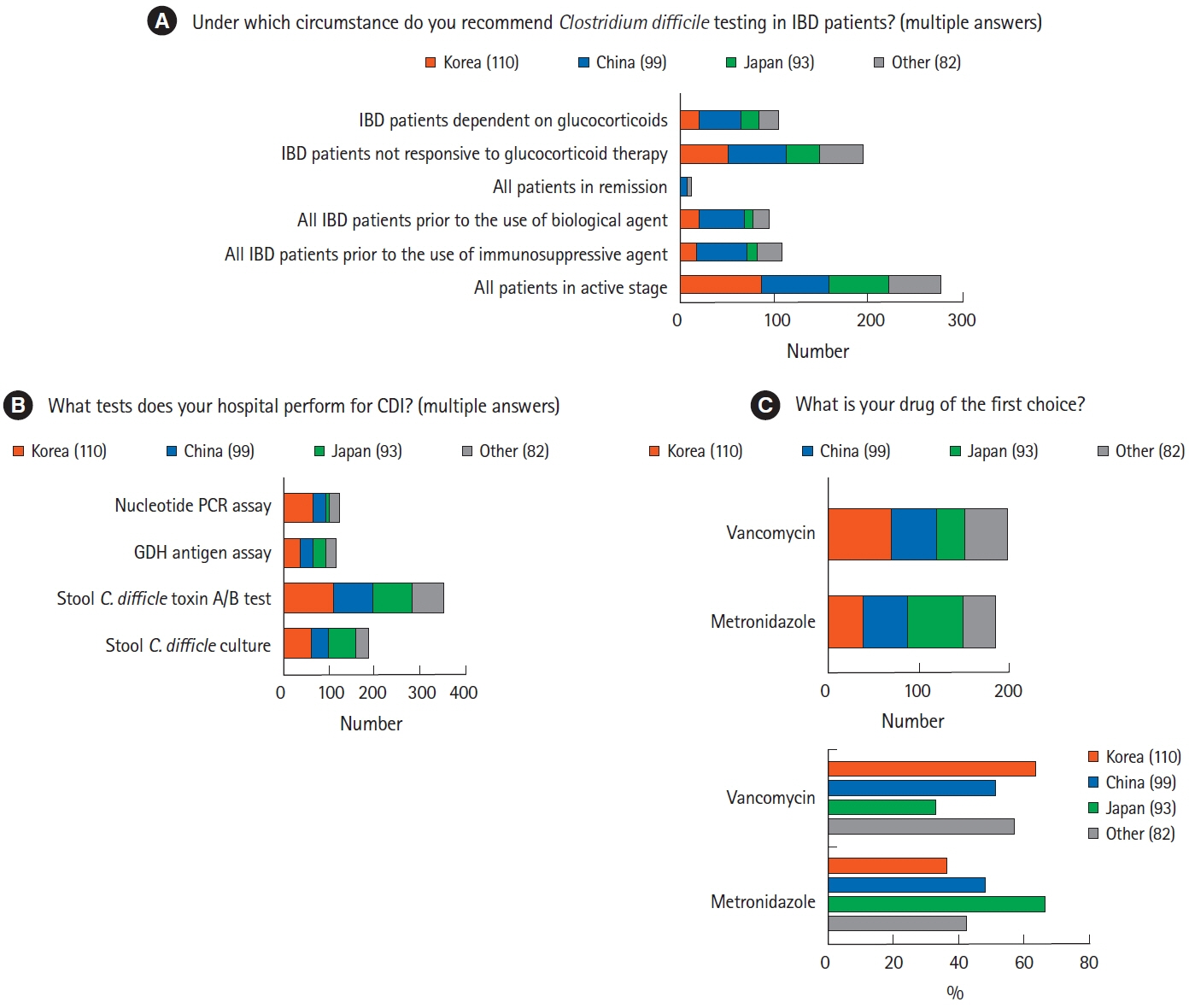
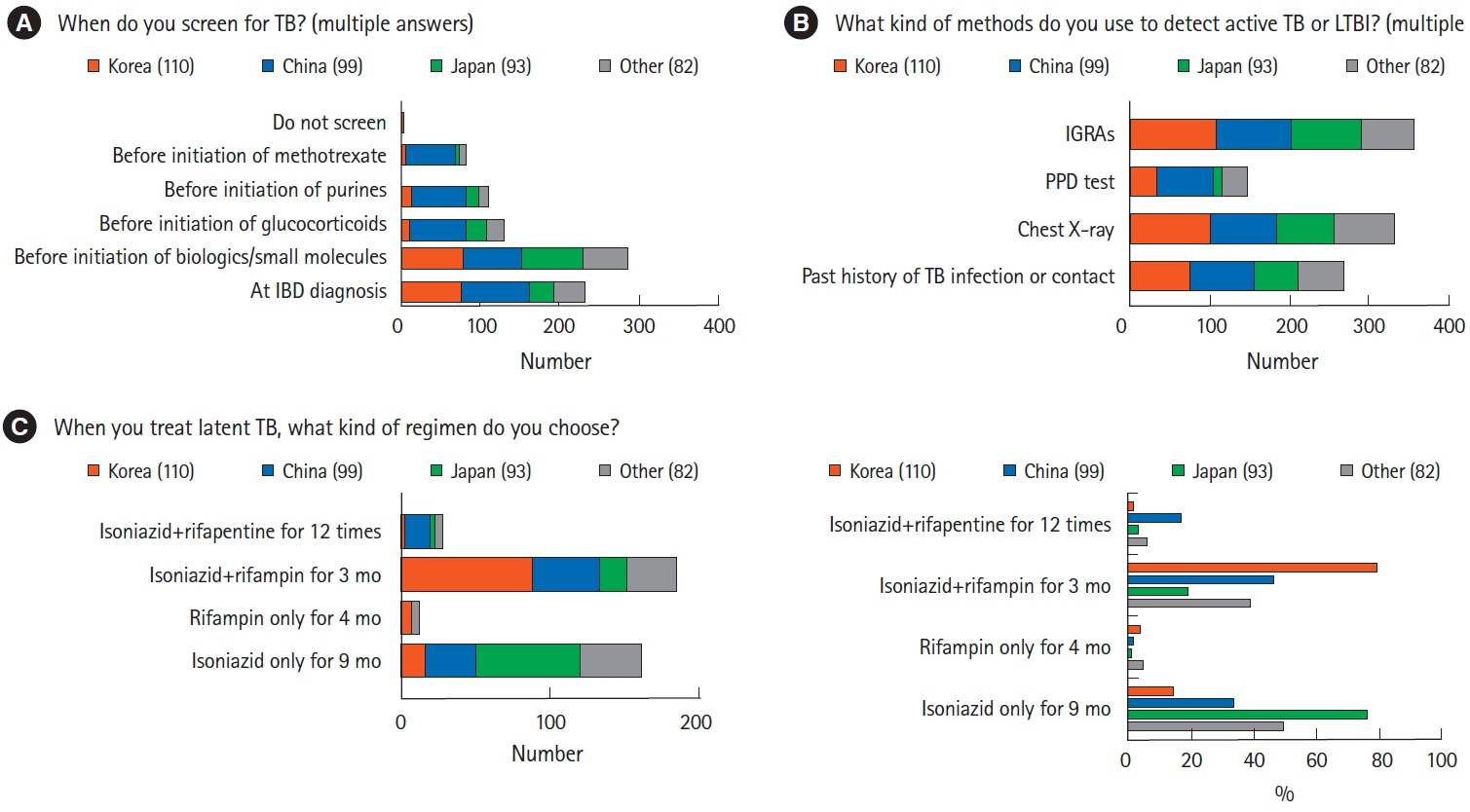
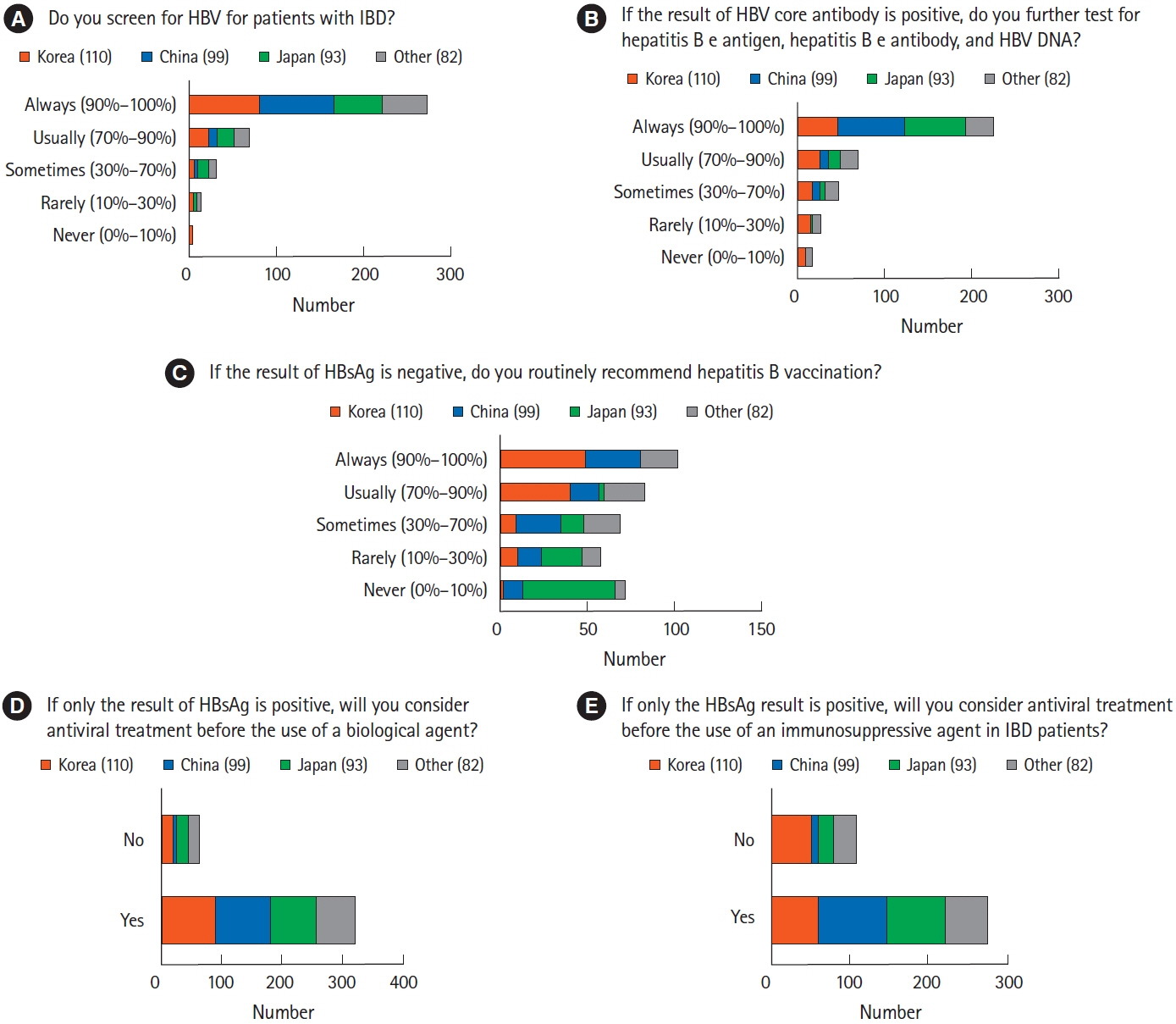
A total of 384 physicians responded to the questionnaire. The majority of Korean (n=70, 63.6%) and Chinese (n=51, 51.5%) physicians preferred vancomycin to metronidazole in the treatment of Clostridium difficile infection, whereas more than half of the Japanese physicians (n=62, 66.7%) preferred metronidazole. Physicians in Korea (n=88, 80.0%) and China (n=46, 46.5%) preferred a 3-month course of isoniazid and rifampin to treat latent tuberculosis infection, whereas most physicians in Japan (n=71, 76.3%) favored a 9-month course of isoniazid. Most Korean physicians (n=89, 80.9%) recommended hepatitis B virus (HBV) vaccination in patients lacking HBV surface antigen, whereas more than half of Japanese physicians (n=53, 57.0%) did not consider vaccination.
Conclusions
Differences in the diagnosis, prevention, and management of infections in patients with IBD across countries/regions reflect different prevalence rates of infectious diseases. This survey may broaden understanding of the real-world clinical settings across Asian countries/regions and provide information for establishing practical guidelines to manage patients with IBD.
Keyword
Figure
Cited by 1 articles
-
Diagnosis, management, and prevention of infectious complications in inflammatory bowel disease: variations among Asian countries
Ji Eun Baek, Sung Wook Hwang
Intest Res. 2023;21(3):277-279. doi: 10.5217/ir.2023.00076.
Reference
-
1. Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008; 14:354–377.
Article2. Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. 2022; 20:159–164.
Article3. Ye BD, Hong SN, Seo SI, et al. Changes in the long-term prognosis of Crohn’s disease between 1986 and 2015: the population-based Songpa-Kangdong inflammatory bowel disease cohort study. Gut Liver. 2022; 16:216–227.
Article4. Annese V, Duricova D, Gower-Rousseau C, Jess T, Langholz E. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the Epidemiology Committee of ECCO. J Crohns Colitis. 2016; 10:216–225.
Article5. Barberio B, Savarino EV, Card T, et al. Incidence comparison of adverse events in patients with inflammatory bowel disease receiving different biologic agents: retrospective long-term evaluation. Intest Res. 2022; 20:114–123.
Article6. Singh A, Mahajan R, Kedia S, et al. Use of thiopurines in inflammatory bowel disease: an update. Intest Res. 2022; 20:11–30.
Article7. Hibi T, Kamae I, Pinton P, et al. Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis. Intest Res. 2021; 19:53–61.
Article8. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article9. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018; 113:481–517.
Article10. Kucharzik T, Ellul P, Greuter T, et al. ECCO Guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021; 15:879–913.
Article11. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article12. Giri S, Agrawal D, Afzalpurkar S, et al. Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res. 2023; 21:392–405.
Article13. Lee JM, Wei SC, Lee KM, et al. Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver. 2022; 16:396–403.
Article14. Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. J Gastroenterol Hepatol. 2019; 34:1296–1315.
Article15. Kang EA, Cheon JH. Antiviral prophylaxis against hepatitis B virus in patients treated with anti-tumor necrosis factor α agents for inflammatory bowel disease. Gut Liver. 2022; 16:501–502.
Article16. Song HK, Lee KM, Jung SA, et al. Quality of care in inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:240–247.
Article17. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:224–230.
Article18. Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:231–239.
Article19. Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020: reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021; 113(Suppl 1):S7–S12.20. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018; 67:1–31.
Article21. Lernout T, Hendrickx G, Vorsters A, Mosina L, Emiroglu N, Van Damme P. A cohesive European policy for hepatitis B vaccination, are we there yet? Clin Microbiol Infect. 2014; 20 Suppl 5:19–24.
Article22. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468.
Article23. Cvetković RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005; 65:859–878.24. Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication). Intest Res. 2018; 16:178–193.25. Tang YM, Stone CD. Clostridium difficile infection in inflammatory bowel disease: challenges in diagnosis and treatment. Clin J Gastroenterol. 2017; 10:112–123.
Article26. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008; 103:1443–1450.
Article27. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017; 15:7–37.
Article28. Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009; 47:3211–3217.
Article29. Bélanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG. Rapid detection of Clostridium difficile in feces by real-time PCR. J Clin Microbiol. 2003; 41:730–734.30. Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021; 116:1124–1147.
Article31. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007; 45:302–307.
Article32. Yamagishi Y, Mikamo H. Recent epidemiology of Clostridium difficile infection in Japan. Jpn J Antibiot. 2015; 68:345–358.33. Allegretti JR, Kelly CR, Grinspan A, Mullish BH, Kassam Z, Fischer M. Outcomes of fecal microbiota transplantation in patients with inflammatory bowel diseases and recurrent Clostridioides difficile infection. Gastroenterology. 2020; 159:1982–1984.
Article34. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis. 2020; 26:1415–1420.
Article35. Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019; 54:1900655.
Article36. Zhu S, Xia L, Yu S, Chen S, Zhang J. The burden and challenges of tuberculosis in China: findings from the Global Burden of Disease Study 2015. Sci Rep. 2017; 7:14601.
Article37. World Health Organization (WHO). Global tuberculosis report 2020 [Internet]. c2020 [cited 2023 May 18]. https://www.who.int/publications/i/item/9789240013131.38. Limsrivilai J, Pausawasdi N. Intestinal tuberculosis or Crohn’s disease: a review of the diagnostic models designed to differentiate between these two gastrointestinal diseases. Intest Res. 2021; 19:21–32.
Article39. Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 27:19–30.
Article40. Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012; 18:1057–1063.
Article41. Riestra S, Taxonera C, Zabana Y, et al. Performance of screening strategies for latent tuberculosis infection in patients with inflammatory bowel disease: results from the ENEIDA registry of GETECCU. J Clin Med. 2022; 11:3915.
Article42. Lee SH. Diagnosis and treatment of latent tuberculosis infection: the updated 2017 Korean Guidelines. Korean J Med. 2018; 93:509–517.
Article43. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019; 16:57–73.
Article44. Merican I, Guan R, Amarapuka D, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000; 15:1356–1361.
Article45. Sagami S, Kobayashi T, Hibi T. Prevention of infectious diseases due to immunosuppression and vaccinations in Asian patients with inflammatory bowel disease. Inflamm Intest Dis. 2018; 3:1–10.
Article46. Ujiie M, Sasaki K, Yoshikawa N, Enami T, Shobayashi T. Introduction of a hepatitis B vaccine into the national routine immunisation programme of Japan. Lancet Infect Dis. 2016; 16:1325.
Article47. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article48. Yang H, Ran Z, Jin M, Qian JM. Current status of opportunistic infection in inflammatory bowel disease patients in Asia: a questionnaire-based multicenter study. Gut Liver. 2022; 16:726–735.
Article49. Pan Z, Zhang J, Bu Q, et al. The gap between global tuberculosis incidence and the first milestone of the WHO end tuberculosis strategy: an analysis based on the global burden of disease 2017 database. Infect Drug Resist. 2020; 13:1281–1286.50. Ginzberg D, Wong RJ, Gish R. Global HBV burden: guesstimates and facts. Hepatol Int. 2018; 12:315–329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul
- Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization for Crohn's and Colitis (AOCC) meeting in Seoul
- Treatment of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Erratum: Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn's and Colitis (AOCC) meeting in Seoul