Intest Res.
2023 Jul;21(3):339-352. 10.5217/ir.2022.00135.
Treatment of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 3Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
- 5Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Japan
- 6Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Inflammatory Bowel Disease Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2544757
- DOI: http://doi.org/10.5217/ir.2022.00135
Abstract
- Background/Aims
As the characteristics of inflammatory bowel disease (IBD) differ between Asians and Westerners, it is necessary to determine adequate therapeutic strategy for Asian IBD patients. We evaluated the current treatment of IBD in Asian countries/regions using a web-based survey.
Methods
The Korean Association for the Study of Intestinal Diseases conducted a multinational web-based survey for current IBD care in Asia between September 16, 2020, and November 13, 2020.
Results
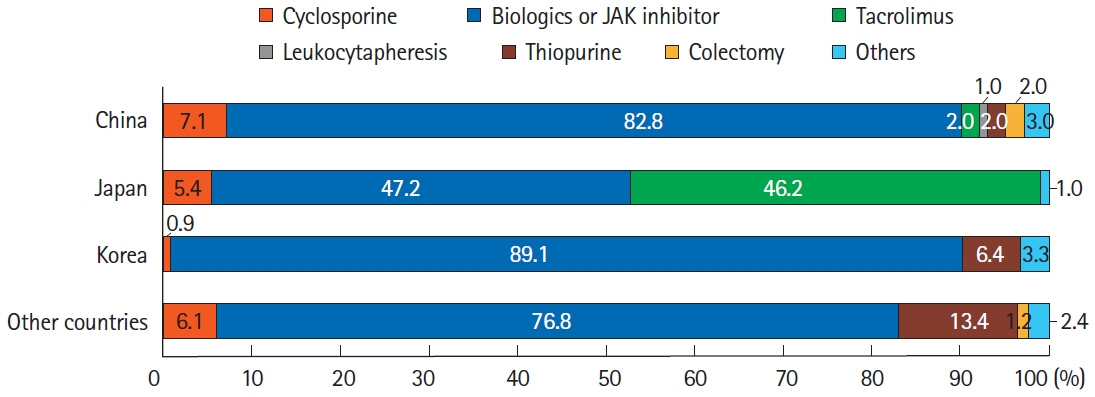
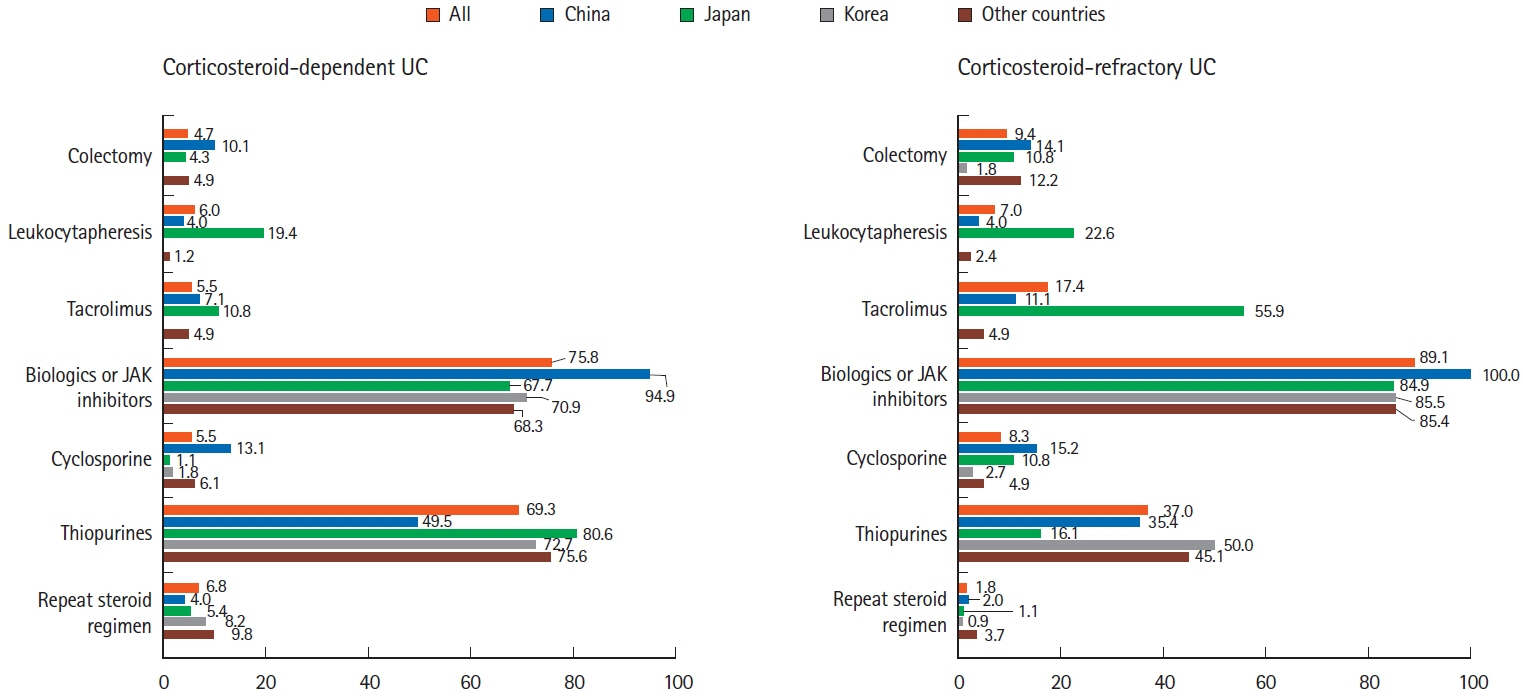
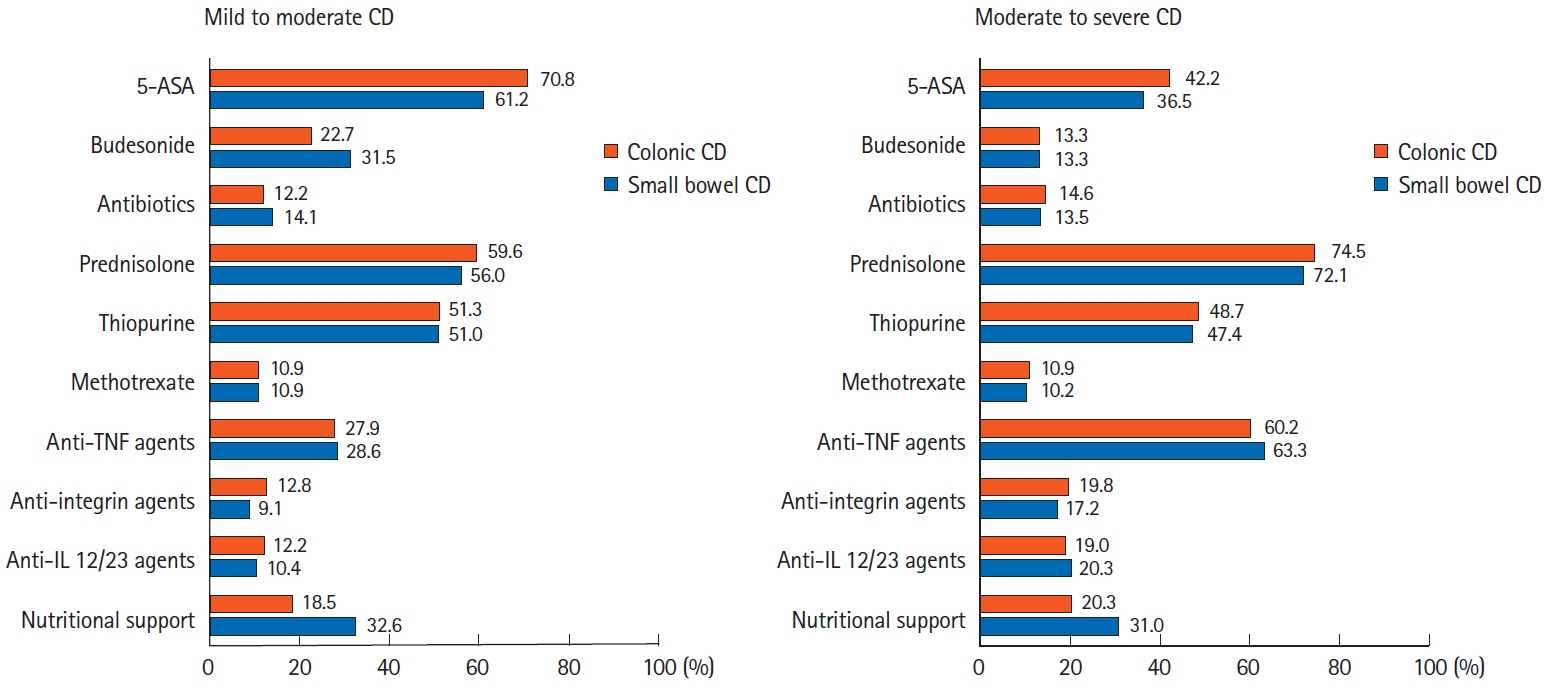
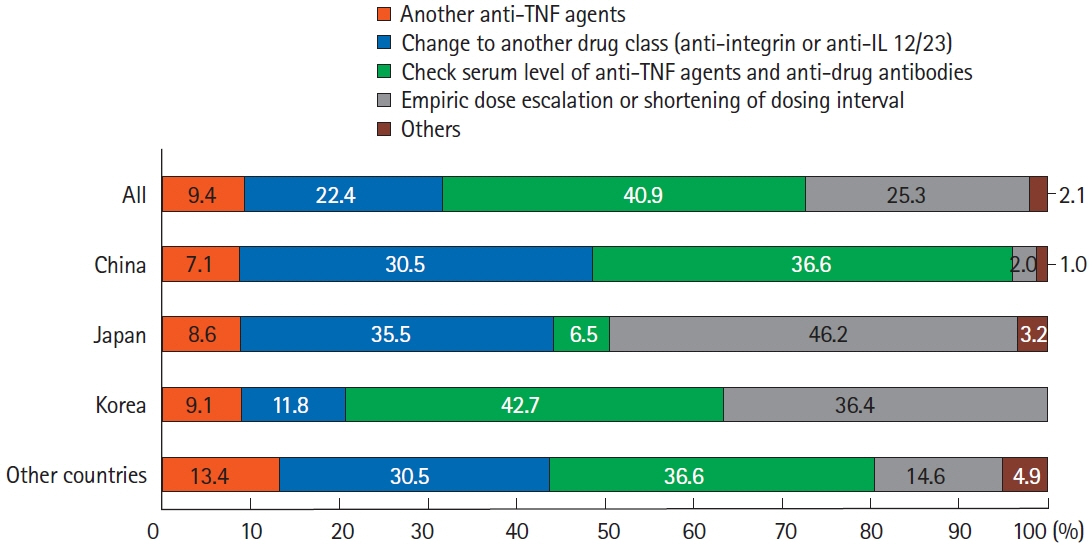
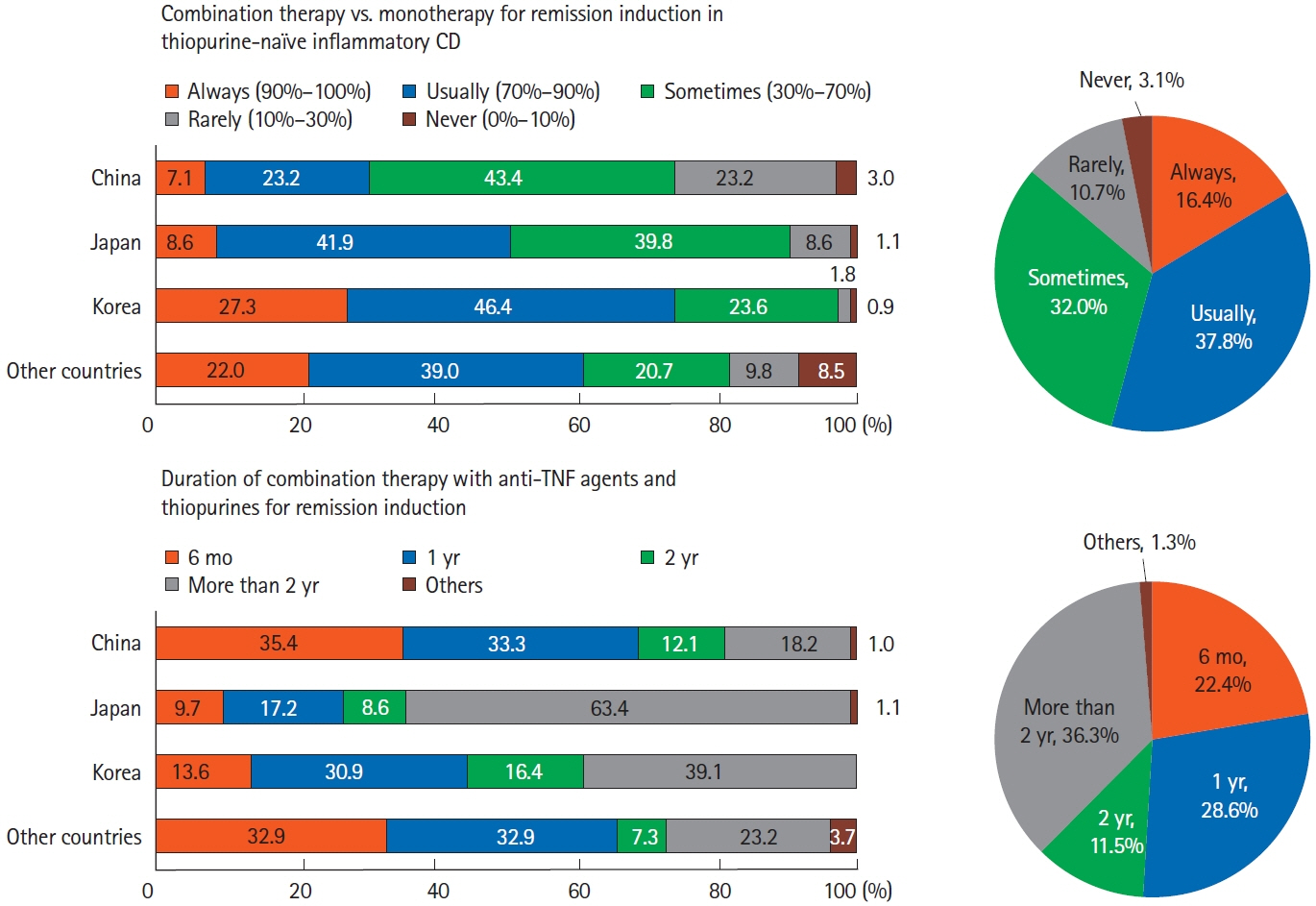
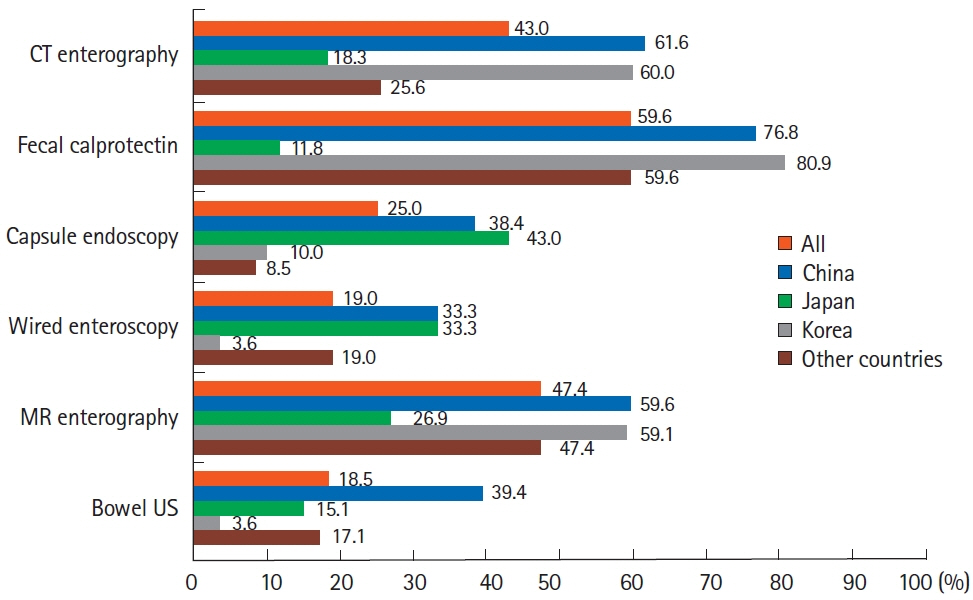
A total of 384 doctors treating IBD patients from 24 Asian countries/regions responded to the survey. Anti-tumor necrosis factor (TNF) agents, anti-integrins, and anti-interleukin-12/23 agents were available for use by 93.8%, 72.1%, and 70.1% of respondents in Asian countries/regions. Compared with a previous survey performed in 2014, an increased tendency for treatment with biologics, including anti-TNF agents, was observed. In the treatment of corticosteroid-refractory acute severe ulcerative colitis, 72.1% of respondents chose anti-TNF agents, followed by tacrolimus (11.7%). In the treatment of corticosteroid-refractory Crohn’s disease, 90.4% chose anti-TNF agents, followed by thiopurines (53.1%), anti-interleukin-12/23 agents (39.3%), and anti-integrin agents (35.7%). In the treatment of Crohn’s disease patients refractory to anti-TNF agents, the most preferred strategy was to measure serum levels of anti-TNF and anti-drug antibodies (40.9%), followed by empiric dose escalation or shortening of dosing intervals (25.3%).
Conclusions
Although there were some differences, treatment strategies for patients with IBD were mostly similar among Asian doctors. Based on the therapeutic outcomes, it is necessary to identify the most appropriate therapeutic strategy for Asian IBD patients.
Keyword
Figure
Cited by 1 articles
-
How have treatment patterns for patients with inflammatory bowel disease changed in Asian countries?
Jihye Park
Intest Res. 2023;21(3):275-276. doi: 10.5217/ir.2023.00061.
Reference
-
1. Yang Y, Owyang C, Wu GD. East meets west: the increasing incidence of inflammatory bowel disease in Asia as a paradigm for environmental effects on the pathogenesis of immune-mediated disease. Gastroenterology. 2016; 151:e1–e5.
Article2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article3. Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17:54–62.
Article4. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020; 35:380–389.
Article5. Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. 2016; 22:1954–1960.6. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019; 13:1410–1417.
Article7. Yen HH, Hsu TC, Chen MW, Su PY, Chen YY. Clinical features and treatment of inflammatory bowel disease in a low-incidence area: a hospital-based retrospective cohort study in Taiwan. Medicine (Baltimore). 2021; 100:e25090.8. Song EM, Lee HS, Kim YJ, et al. Incidence and outcomes of perianal disease in an Asian population with Crohn’s disease: a nationwide population-based study. Dig Dis Sci. 2020; 65:1189–1196.
Article9. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013; 28:1148–1153.
Article10. Shi HY, Levy AN, Trivedi HD, Chan FK, Ng SC, Ananthakrishnan AN. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol. 2018; 16:190–197.
Article11. Shah SC, Khalili H, Chen CY, et al. Sex-based differences in the incidence of inflammatory bowel diseases-pooled analysis of population-based studies from the Asia-Pacific region. Aliment Pharmacol Ther. 2019; 49:904–911.
Article12. Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015; 47:979–986.
Article13. Hong M, Ye BD, Yang SK, et al. Immunochip meta-analysis of inflammatory bowel disease identifies three novel loci and four novel associations in previously reported loci. J Crohns Colitis. 2018; 12:730–741.
Article14. Jung S, Ye BD, Lee HS, et al. Identification of three novel susceptibility loci for inflammatory bowel disease in Koreans in an extended genome-wide association study. J Crohns Colitis. 2021; 15:1898–1907.
Article15. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article16. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018; 113:481–517.
Article17. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020; 14:4–22.18. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1450–1461.
Article19. Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019; 156:748–764.
Article20. Feuerstein JD, Ho EY, Shmidt E, et al. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology. 2021; 160:2496–2508.21. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article22. Park JJ, Yang SK, Ye BD, et al. Second Korean guidelines for the management of Crohn’s disease. Intest Res. 2017; 15:38–67.
Article23. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017; 15:7–37.
Article24. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article25. Wei SC, Chang TA, Chao TH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017; 15:266–284.
Article26. Wei SC, Chang TA, Chao TH, et al. Management of Crohn’s disease in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017; 15:285–310.
Article27. Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:231–239.
Article28. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:224–230.
Article29. Song HK, Lee KM, Jung SA, et al. Quality of care in inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:240–247.
Article30. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19 Suppl A:5A–36A.
Article31. Targownik LE, Tennakoon A, Leung S, Lix LM, Singh H, Bernstein CN. Temporal trends in initiation of therapy with tumor necrosis factor antagonists for patients with inflammatory bowel disease: a population-based analysis. Clin Gastroenterol Hepatol. 2017; 15:1061–1070.
Article32. Brunet E, Vela E, Melcarne L, et al. Time trends of Crohn’s disease in Catalonia from 2011 to 2017. increasing use of biologics correlates with a reduced need for surgery. J Clin Med. 2020; 9:2896.
Article33. Song EM, Yang SK. Natural history of inflammatory bowel disease: a comparison between the East and the West. Intest Res. 2022; 20:418–430.
Article34. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012; 380:1909–1915.
Article35. Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018; 67:237–243.
Article36. Song EM, Oh EH, Hwang SW, et al. Comparison of outcomes of cyclosporine A and infliximab for steroid-refractory acute severe ulcerative colitis. J Gastroenterol Hepatol. 2021; 36:2463–2470.
Article37. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article38. Chinese Cooperative Group for The Study On IBD; Chinese Society of Gastroenterology, Ouyang Q, et al. Consensus on the management of inflammatory bowel disease in China in 2007. J Dig Dis. 2008; 9:52–62.
Article39. Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn’s disease: the third IBD? Gut. 2017; 66:362–381.
Article40. Summers RW, Switz DM, Sessions JT Jr, et al. National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology. 1979; 77(4 Pt 2):847–869.
Article41. Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn’s Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984; 86:249–266.
Article42. Noureldin M, Cohen-Mekelburg S, Mahmood A, et al. Trends of 5-aminosalicylate medication use in patients with Crohn disease. Inflamm Bowel Dis. 2021; 27:516–521.
Article43. Hida N, Nakamura S, Hahm KB, et al. A questionnaire-based survey on the diagnosis and management of inflammatory bowel disease in East Asian countries in 2012. Digestion. 2014; 89:88–103.
Article44. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010; 105:1133–1139.
Article45. Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016; 14:251–258.
Article46. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017; 153:827–834.
Article47. Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017; 153:835–857.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul
- Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization for Crohn's and Colitis (AOCC) meeting in Seoul
- Vaccination in patients with inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
- Infectious complications in patients with inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting