Clin Exp Otorhinolaryngol.
2023 May;16(2):184-197. 10.21053/ceo.2022.01760.
Mitochondrial Ribosomal Protein L14 Promotes Cell Growth and Invasion by Modulating Reactive Oxygen Species in Thyroid Cancer
- Affiliations
-
- 1Department of Medical Science, Chungnam National University College of Medicine, Daejeon, Korea
- 2Department of Otolaryngology-Head and Neck Surgery, Chungnam National University College of Medicine, Daejeon, Korea
- 3Department of Radiation Oncology, Chungnam National University Sejong Hospital, Sejong, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- KMID: 2542360
- DOI: http://doi.org/10.21053/ceo.2022.01760
Abstract
Objectives
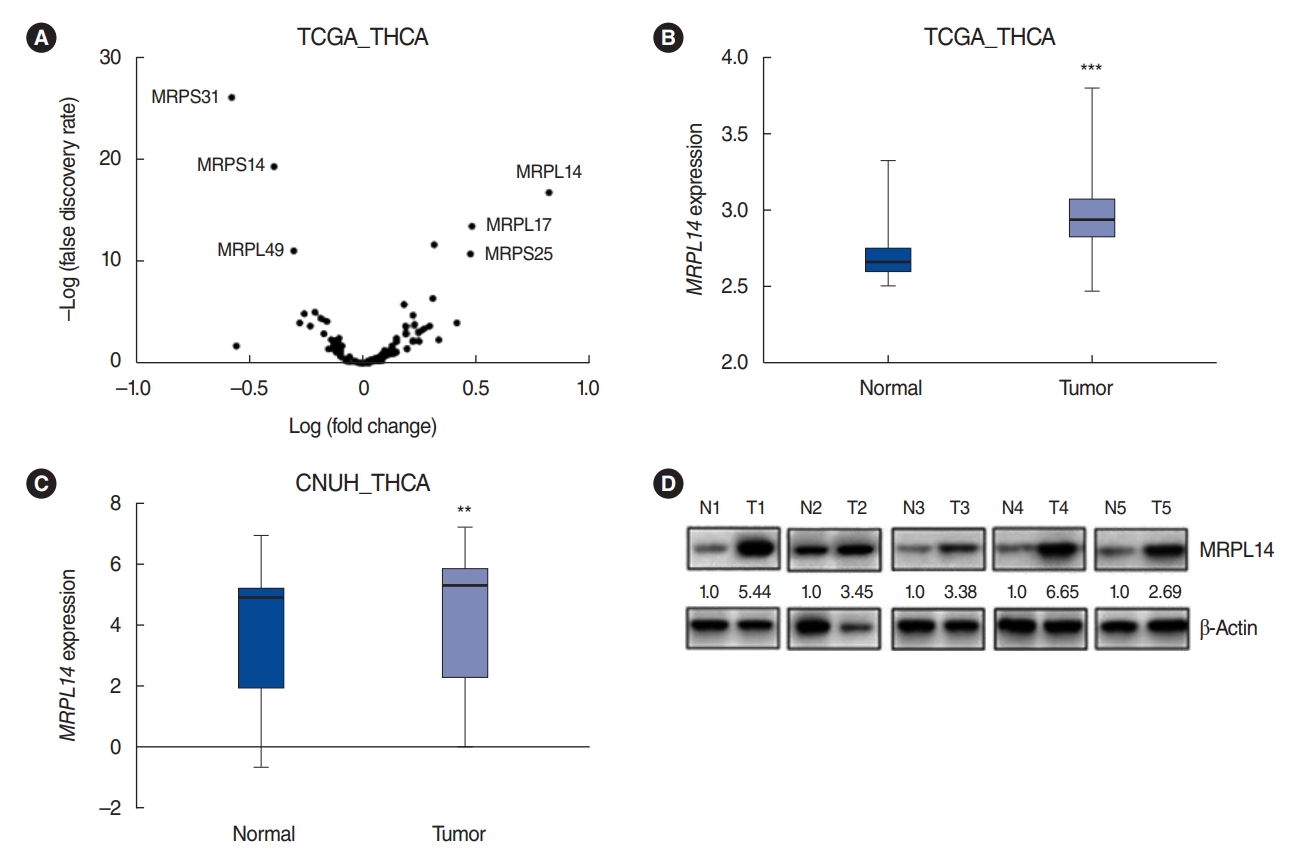
. The mitochondrial ribosomal protein L14 (MRPL14) is encoded by a nuclear gene and participates in mitochondrial protein translation. In this study, we aimed to investigate the role of MRPL14 in thyroid cancer.
Methods
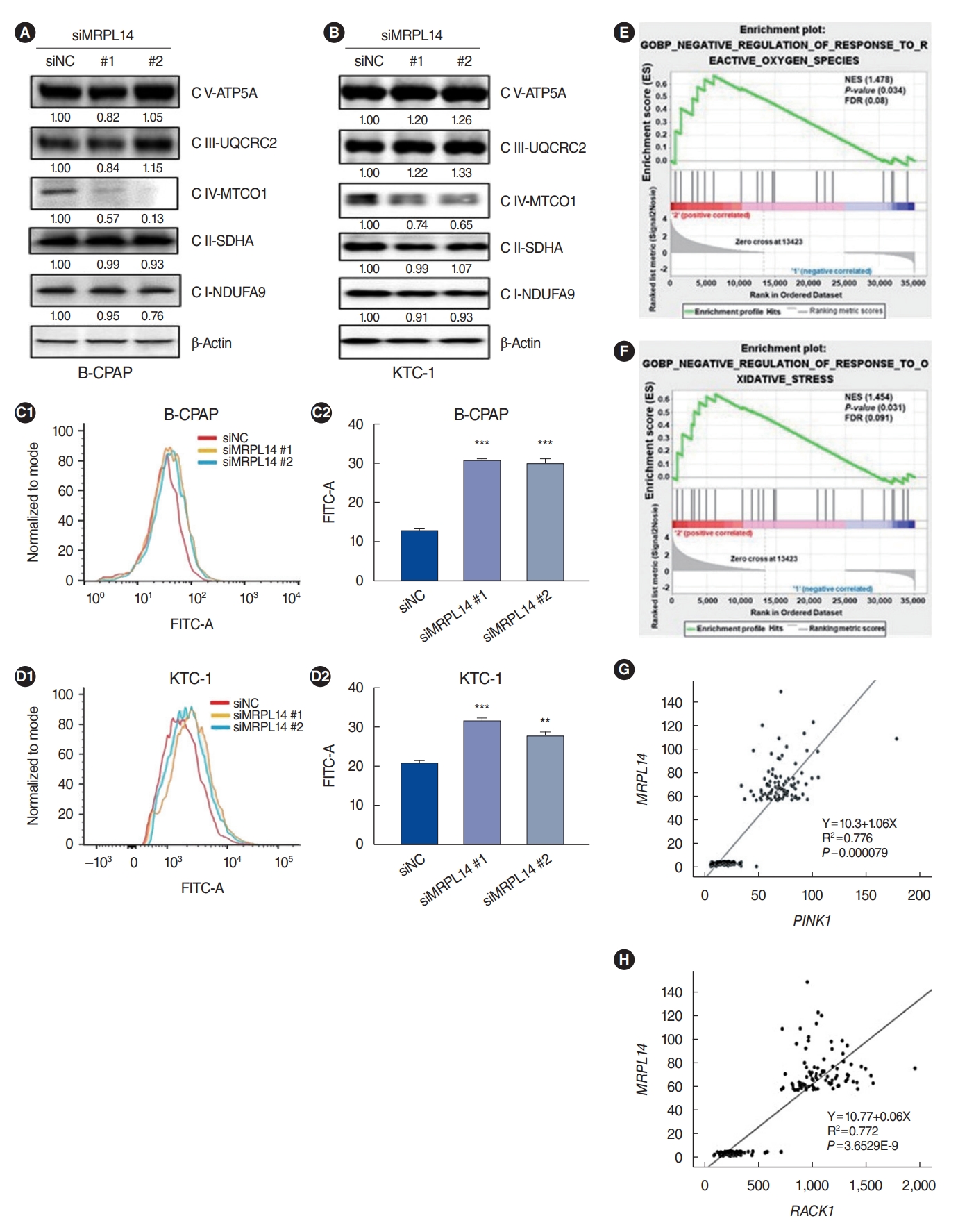
. We investigated the association between MRPL14 expression and clinicopathological features using The Cancer Genome Atlas (TCGA) and Chungnam National University Hospital (CNUH) databases. Functional studies of MRPL14, including proliferation, migration, invasion, mitochondrial oxidative phosphorylation and reactive oxygen species (ROS) production, were performed in papillary thyroid cancer (PTC) cell lines (B-CPAP and KTC-1).
Results
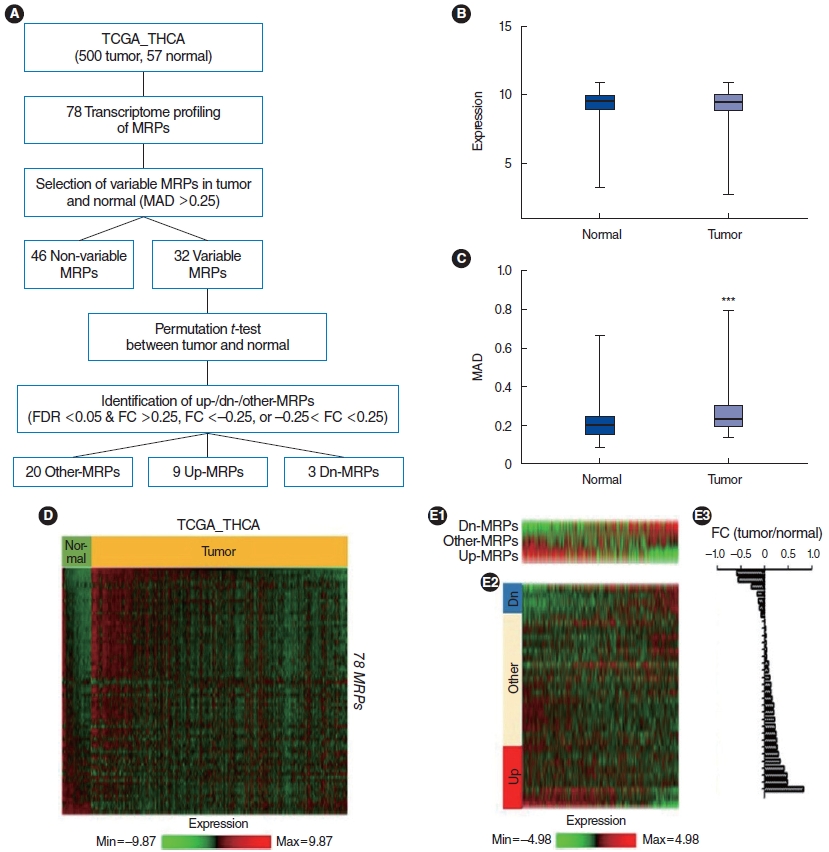
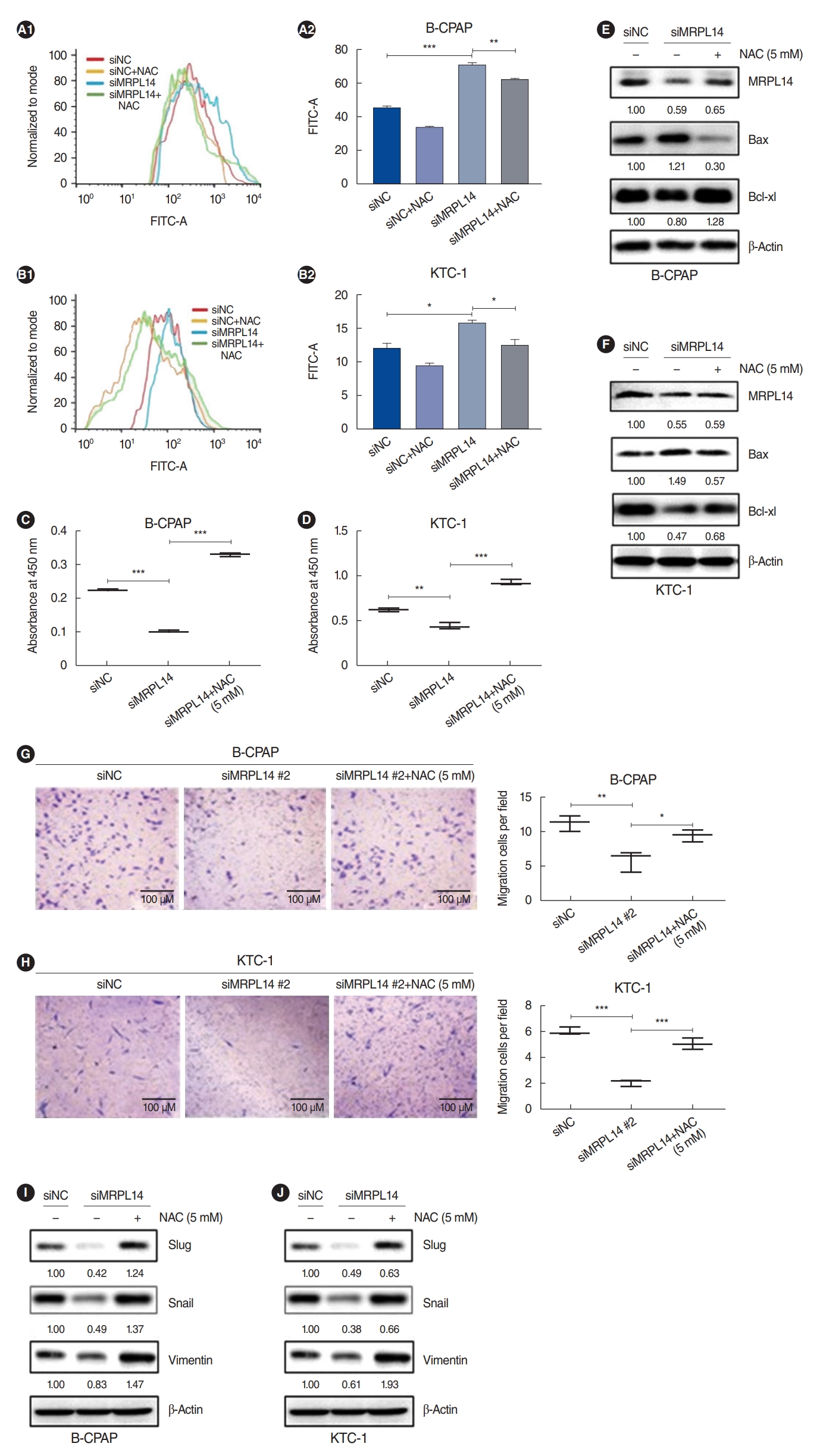
. Based on the TCGA dataset, PTC tissues lost mitochondrial integrity and showed dysregulated expression of overall mitoribosomal proteins (MRPs) compared with normal thyroid tissues. Of 78 MRPs, MRPL14 was highly expressed in thyroid cancer tissues. MRPL14 overexpression was significantly associated with advanced tumor stage, extrathyroidal extension, and lymph node metastasis. MRPL14 increased cell proliferation of thyroid cancer and promoted cell migration via epithelial-mesenchymal transition-related proteins. Moreover, MRPL14 knockdown reduced the expression of oxidative phosphorylation complex IV (MTCO1) and increased the accumulation of ROS. Cotreatment with a ROS scavenger restored cell proliferation and migration, which had been reduced by MRPL14 knockdown, implying that ROS functions as a key regulator of the oncogenic effects of MRPL14 in thyroid cancer cells.
Conclusion
. Our findings indicate that MRPL14 may promote cell growth, migration, and invasion by modulating ROS in thyroid cancer cells.
Keyword
Figure
Reference
-
1. Ancker OV, Kruger M, Wehland M, Infanger M, Grimm D. Multikinase inhibitor treatment in thyroid cancer. Int J Mol Sci. 2019; Dec. 21(1):10.
Article2. Ahn SH. Usage and diagnostic yield of fine-needle aspiration cytology and core needle biopsy in thyroid nodules: a systematic review and meta-analysis of literature published by Korean authors. Clin Exp Otorhinolaryngol. 2021; Feb. 14(1):116–30.
Article3. Park SJ, Kang YE, Kim JH, Park JL, Kim SK, Baek SW, et al. Transcriptomic analysis of papillary thyroid cancer: a focus on immunesubtyping, oncogenic fusion, and recurrence. Clin Exp Otorhinolaryngol. 2022; May. 15(2):183–93.
Article4. Yi JW, Ha SY, Jee HG, Kim K, Kim SJ, Chai YJ, et al. Induction of the BRAFV600E mutation in thyroid cells leads to frequent hypermethylation. Clin Exp Otorhinolaryngol. 2022; Aug. 15(3):273–82.
Article5. Zheng CM, Ji YB, Song CM, Ge MH, Tae K. Number of metastatic lymph nodes and ratio of metastatic lymph nodes to total number of retrieved lymph nodes are risk factors for recurrence in patients with clinically node negative papillary thyroid carcinoma. Clin Exp Otorhinolaryngol. 2018; Mar. 11(1):58–64.
Article6. Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008; Jun. 37(2):525–38.
Article7. Tang P, Dang H, Huang J, Xu T, Yuan P, Hu J, et al. NADPH oxidase NOX4 is a glycolytic regulator through mROS-HIF1α axis in thyroid carcinomas. Sci Rep. 2018; Oct. 8(1):15897.
Article8. Kim HJ, Maiti P, Barrientos A. Mitochondrial ribosomes in cancer. Semin Cancer Biol. 2017; Dec. 47:67–81.
Article9. Yan C, Duanmu X, Zeng L, Liu B, Song Z. Mitochondrial DNA: distribution, mutations, and elimination. Cells. 2019; Apr. 8(4):379.
Article10. Huang G, Li H, Zhang H. Abnormal expression of mitochondrial ribosomal proteins and their encoding genes with cell apoptosis and diseases. Int J Mol Sci. 2020; Nov. 21(22):8879.
Article11. Sorensen KM, Meldgaard T, Melchjorsen CJ, Fridriksdottir AJ, Pedersen H, Petersen OW, et al. Upregulation of Mrps18a in breast cancer identified by selecting phage antibody libraries on breast tissue sections. BMC Cancer. 2017; Jan. 17(1):19.
Article12. Koc EC, Haciosmanoglu E, Claudio PP, Wolf A, Califano L, Friscia M, et al. Impaired mitochondrial protein synthesis in head and neck squamous cell carcinoma. Mitochondrion. 2015; Sep. 24:113–21.
Article13. Wang Z, Li J, Long X, Jiao L, Zhou M, Wu K. MRPS16 facilitates tumor progression via the PI3K/AKT/Snail signaling axis. J Cancer. 2020; Feb. 11(8):2032–43.
Article14. Oviya RP, Gopal G, Shirley SS, Sridevi V, Jayavelu S, Rajkumar T. Mitochondrial ribosomal small subunit proteins (MRPS) MRPS6 and MRPS23 show dysregulation in breast cancer affecting tumorigenic cellular processes. Gene. 2021; Jul. 790:145697.
Article15. Fung S, Nishimura T, Sasarman F, Shoubridge EA. The conserved interaction of C7orf30 with MRPL14 promotes biogenesis of the mitochondrial large ribosomal subunit and mitochondrial translation. Mol Biol Cell. 2013; Feb. 24(3):184–93.
Article16. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012; Mar. 7(3):562–78.
Article17. Ferreira JA, Zwinderman AH. On the benjamini-hochberg method. Ann Statist. 2006; Aug. 34:1827–49.
Article18. van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011; Jul-Oct. 728(1-2):23–34.
Article19. Zhang L, Lu P, Yan L, Yang L, Wang Y, Chen J, et al. MRPL35 is upregulated in colorectal cancer and regulates colorectal cancer cell growth and apoptosis. Am J Pathol. 2019; May. 189(5):1105–20.
Article20. Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020; Feb. 52(2):192–203.
Article21. Liu Y, Shi Y. Mitochondria as a target in cancer treatment. MedComm (2020). 2020; Jul. 1(2):129–39.
Article22. Huang Y, Zhou J, Luo S, Wang Y, He J, Luo P, et al. Identification of a fluorescent small-molecule enhancer for therapeutic autophagy in colorectal cancer by targeting mitochondrial protein translocase TIM44. Gut. 2018; Feb. 67(2):307–19.
Article23. Head SA, Shi W, Zhao L, Gorshkov K, Pasunooti K, Chen Y, et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc Natl Acad Sci U S A. 2015; Dec. 112(52):E7276–85.
Article24. Richter U, Lahtinen T, Marttinen P, Myohanen M, Greco D, Cannino G, et al. A mitochondrial ribosomal and RNA decay pathway blocks cell proliferation. Curr Biol. 2013; Mar. 23(6):535–41.
Article25. Yoo YA, Kim MJ, Park JK, Chung YM, Lee JH, Chi SG, et al. Mitochondrial ribosomal protein L41 suppresses cell growth in association with p53 and p27Kip1. Mol Cell Biol. 2005; Aug. 25(15):6603–16.26. Chen YC, Chang MY, Shiau AL, Yo YT, Wu CL. Mitochondrial ribosomal protein S36 delays cell cycle progression in association with p53 modification and p21(WAF1/CIP1) expression. J Cell Biochem. 2007; Mar. 100(4):981–90.
Article27. Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, Salem AF, Tsirigos A, Lamb R, et al. Mitochondria “fuel” breast cancer metabolism: fifteen markers of mitochondrial biogenesis label epithelial cancer cells, but are excluded from adjacent stromal cells. Cell Cycle. 2012; Dec. 11(23):4390–401.
Article28. Lin S, Li Y, Zamyatnin AA Jr, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018; Jul. 233(7):5119–32.
Article29. Elhamamsy AR, Metge BJ, Alsheikh HA, Shevde LA, Samant RS. Ribosome biogenesis: a central player in cancer metastasis and therapeutic resistance. Cancer Res. 2022; Jul. 82(13):2344–53.
Article30. Wang H, Xie B, Kong Y, Tao Y, Yang G, Gao M, et al. Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3. Oncotarget. 2016; Apr. 7(14):18638–50.
Article31. Chen TW, Chang KP, Cheng CC, Chen CY, Hong SW, Sie ZL, et al. Characterization of recurrent relevant genes reveals a novel role of RPL36A in radioresistant oral squamous cell carcinoma. Cancers (Basel). 2021; Nov. 13(22):5623.
Article32. Li J, Feng D, Gao C, Zhang Y, Xu J, Wu M, et al. Isoforms S and L of MRPL33 from alternative splicing have isoform-specific roles in the chemoresponse to epirubicin in gastric cancer cells via the PI3K/AKT signaling pathway. Int J Oncol. 2019; May. 54(5):1591–600.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reactive Oxygen Species Modulator 1 (ROMO1), a New Potential Target for Cancer Diagnosis and Treatment
- Epilepsy, Reactive Oxygen Species and Mitochondria
- Mitochondrial Reactive Oxygen Species Production Mediated by Romo1 Expression
- MS-5, a Naphthalene Derivative, Induces Apoptosis in Human Pancreatic Cancer BxPC-3 Cells by Modulating Reactive Oxygen Species
- Modulation of Reactive Oxygen Species to Overcome 5-Fluorouracil Resistance