Clin Exp Otorhinolaryngol.
2023 May;16(2):165-176. 10.21053/ceo.2022.01522.
Assessment of Esophageal Reconstruction via Bioreactor Cultivation of a Synthetic Scaffold in a Canine Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 2Department of Biomedical Engineering, Inje University, Gimhae, Korea
- 3Department of Nature-Inspired Nanoconvergence Systems, Korea Institute of Machinery and Materials, Daejeon, Korea
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Radiology, Seoul National University, College of Medicine, Seoul, Korea
- 6Department of Pathology, Seoul National University, College of Medicine, Seoul, Korea
- KMID: 2542358
- DOI: http://doi.org/10.21053/ceo.2022.01522
Abstract
Objectives
. Using tissue-engineered materials for esophageal reconstruction is a technically challenging task in animals that requires bioreactor training to enhance cellular reactivity. There have been many attempts at esophageal tissue engineering, but the success rate has been limited due to difficulty in initial epithelialization in the special environment of peristalsis. The purpose of this study was to evaluate the potential of an artificial esophagus that can enhance the regeneration of esophageal mucosa and muscle through the optimal combination of a double-layered polymeric scaffold and a custom-designed mesenchymal stem cell-based bioreactor system in a canine model.
Methods
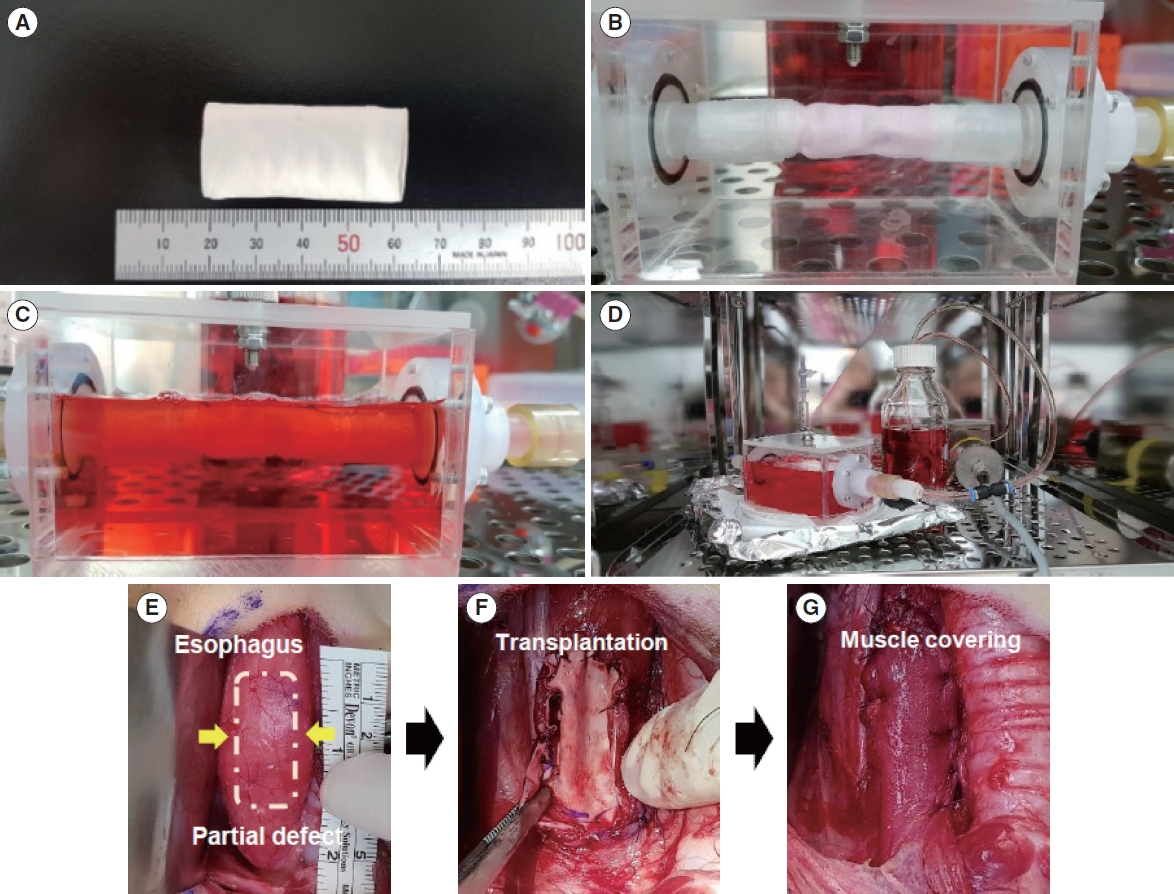
. We fabricated a novel double-layered scaffold as a tissue-engineered esophagus using an electrospinning technique. Prior to transplantation, human-derived mesenchymal stem cells were seeded into the lumen of the scaffold, and bioreactor cultivation was performed to enhance cellular reactivity. After 3 days of cultivation using the bioreactor system, tissue-engineered artificial esophagus was transplanted into a partial esophageal defect (5×3 cm-long resection) in a canine model.
Results
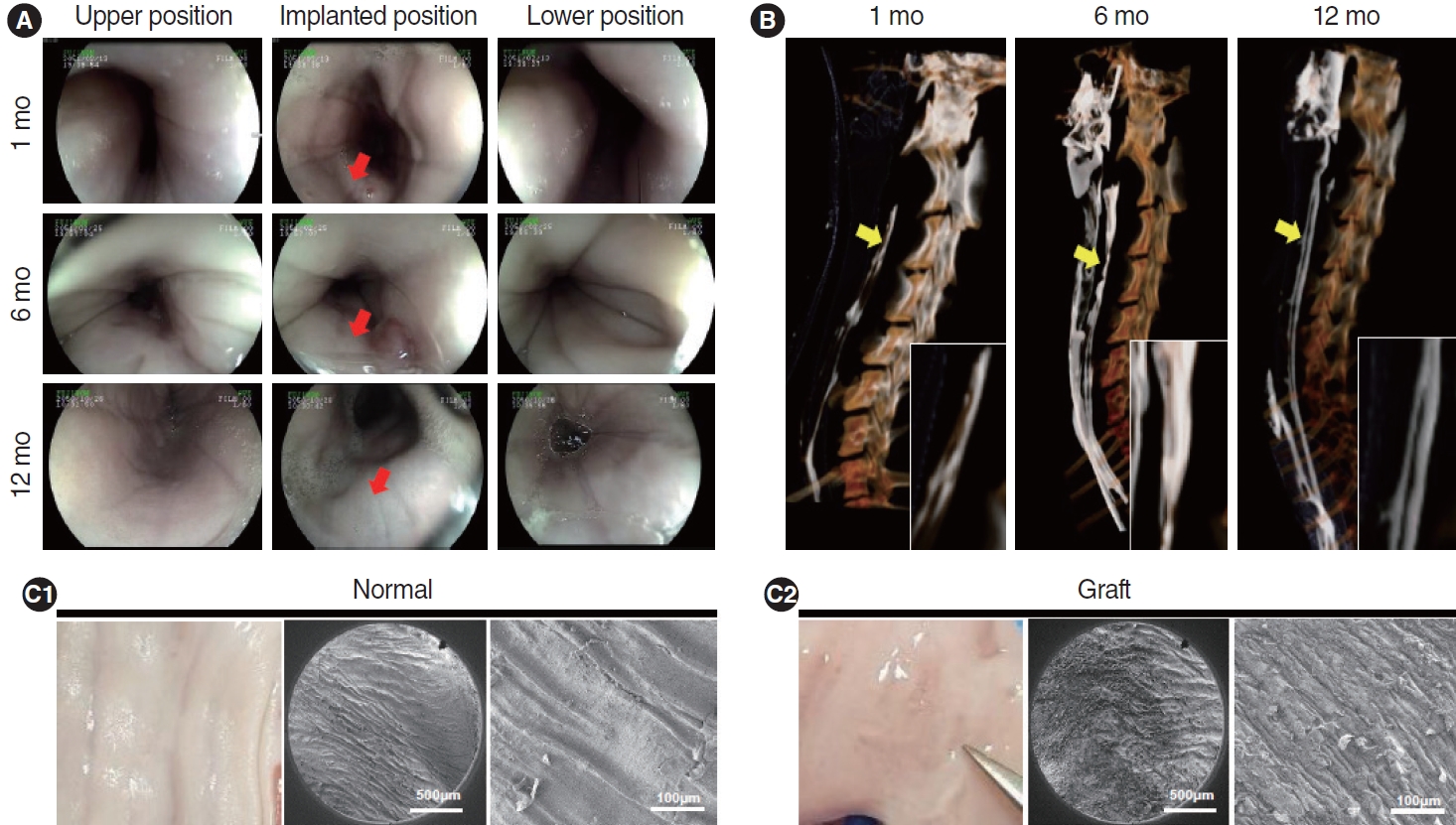
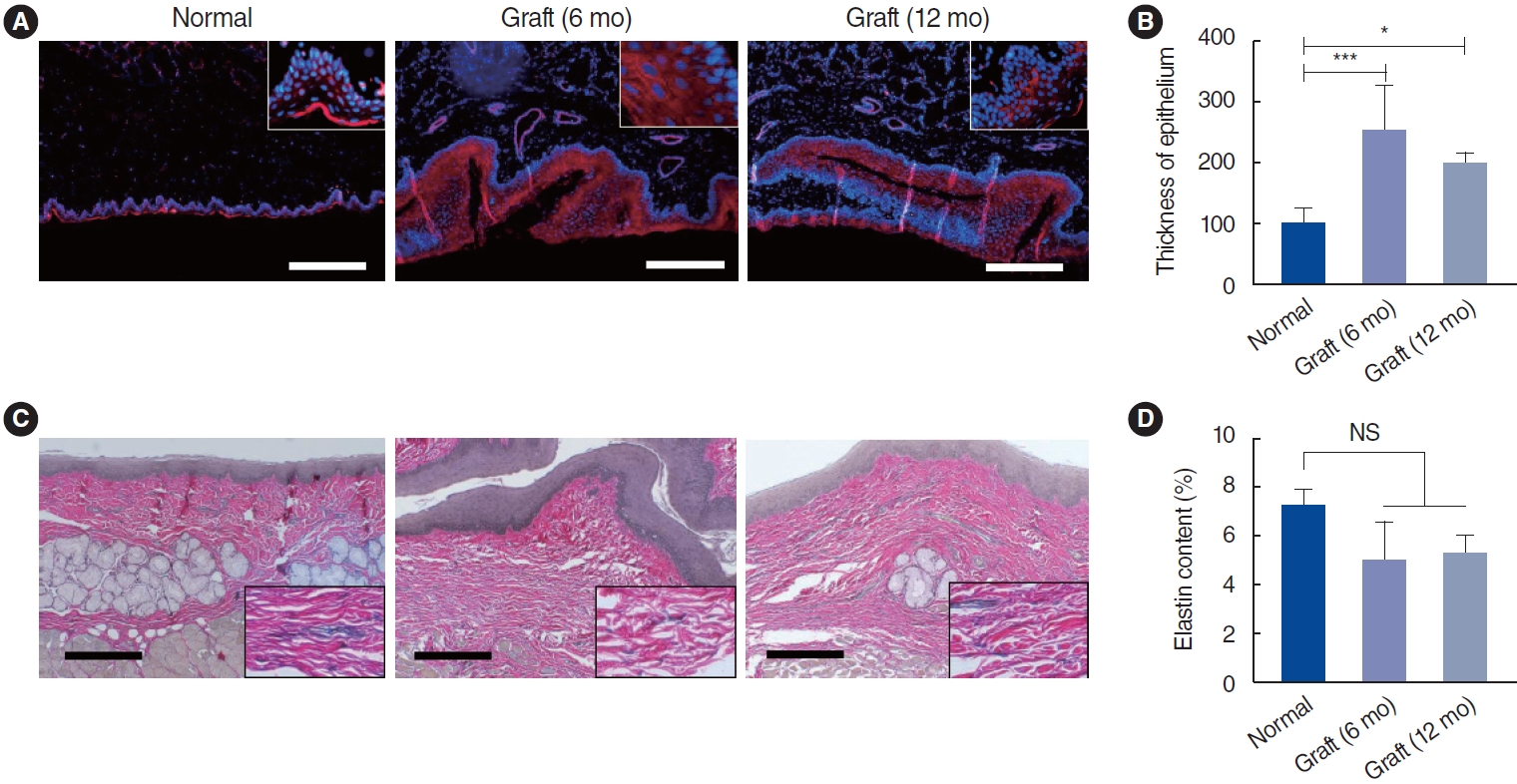
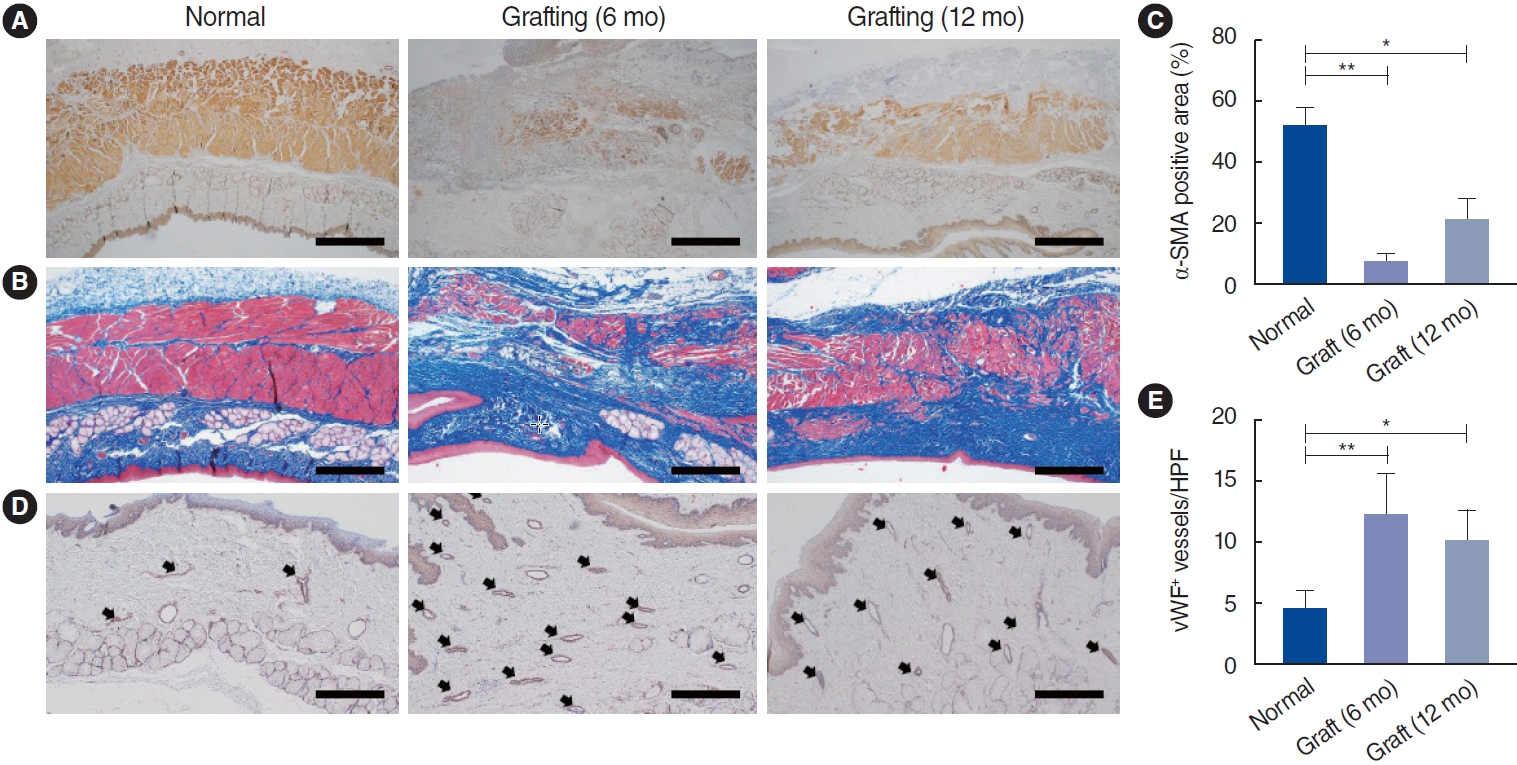
. Scanning electron microscopy (SEM) showed that the electrospun fibers in a tubular scaffold were randomly and circumferentially located toward the inner and outer surfaces. Complete recovery of the esophageal mucosa was confirmed by endoscopic analysis and SEM. Esophagogastroduodenoscopy and computed tomography also showed that there were no signs of leakage or stricture and that there was a normal lumen with complete epithelialization. Significant regeneration of the mucosal layer was observed by keratin-5 immunostaining. Alpha-smooth muscle actin immunostaining showed significantly greater esophageal muscle regeneration at 12 months than at 6 months.
Conclusion
. Custom-designed bioreactor cultured electrospun polyurethane scaffolds can be a promising approach for esophageal tissue engineering.
Figure
Reference
-
1. Luc G, Charles G, Gronnier C, Cabau M, Kalisky C, Meulle M, et al. Decellularized and matured esophageal scaffold for circumferential esophagus replacement: proof of concept in a pig model. Biomaterials. 2018; Aug. 175:1–18.
Article2. Chung EJ. Bioartificial esophagus: where are we now? Adv Exp Med Biol. 2018; Nov. 1064:313–32.
Article3. Irino T, Tsekrekos A, Coppola A, Scandavini CM, Shetye A, Lundell L, et al. Long-term functional outcomes after replacement of the esophagus with gastric, colonic, or jejunal conduits: a systematic literature review. Dis Esophagus. 2017; Dec. 30(12):1–11.
Article4. Totonelli G, Maghsoudlou P, Fishman JM, Orlando G, Ansari T, Sibbons P, et al. Esophageal tissue engineering: a new approach for esophageal replacement. World J Gastroenterol. 2012; Dec. 18(47):6900–7.
Article5. Kim IG, Wu Y, Park SA, Cho H, Choi JJ, Kwon SK, et al. Tissue-engineered esophagus via bioreactor cultivation for circumferential esophageal reconstruction. Tissue Eng Part A. 2019; Nov. 25(21–22):1478–92.
Article6. Park H, Kim IG, Wu Y, Cho H, Shin JW, Park SA, et al. Experimental investigation of esophageal reconstruction with electrospun polyurethane nanofiber and 3D printing polycaprolactone scaffolds using a rat model. Head Neck. 2021; Mar. 43(3):833–48.7. Kim SD, Kim IG, Tran HN, Cho H, Janarthanan G, Noh I, et al. Three-dimensional printed design of antibiotic-releasing esophageal patches for antimicrobial activity prevention. Tissue Eng Part A. 2021; Dec. 27(23–24):1490–502.
Article8. Chung EJ, Ju HW, Park HJ, Park CH. Three-layered scaffolds for artificial esophagus using poly(ɛ-caprolactone) nanofibers and silk fibroin: an experimental study in a rat model. J Biomed Mater Res A. 2015; Jun. 103(6):2057–65.
Article9. Wu Y, Kang YG, Cho H, Kim IG, Chung EJ, Shin JW. Combinational effects of mechanical forces and substrate surface characteristics on esophageal epithelial differentiation. J Biomed Mater Res A. 2019; Mar. 107(3):552–60.
Article10. Wu Y, Kang YG, Kim IG, Kim JE, Lee EJ, Chung EJ, et al. Mechanical stimuli enhance simultaneous differentiation into oesophageal cell lineages in a double-layered tubular scaffold. J Tissue Eng Regen Med. 2019; Aug. 13(8):1394–405.
Article11. Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, et al. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng Part A. 2012; Dec. 18(23–24):2453–65.
Article12. Shah R, Knowles JC, Hunt NP, Lewis MP. Development of a novel smart scaffold for human skeletal muscle regeneration. J Tissue Eng Regen Med. 2016; Feb. 10(2):162–71.
Article13. Heher P, Maleiner B, Pruller J, Teuschl AH, Kollmitzer J, Monforte X, et al. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater. 2015; Sep. 24:251–65.
Article14. Tan JY, Chua CK, Leong KF, Chian KS, Leong WS, Tan LP. Esophageal tissue engineering: an in-depth review on scaffold design. Biotechnol Bioeng. 2012; Jan. 109(1):1–15.
Article15. Chian KS, Leong MF, Kono K. Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol. 2015; Feb. 16(2):e84–92.
Article16. Del Gaudio C, Baiguera S, Ajalloueian F, Bianco A, Macchiarini P. Are synthetic scaffolds suitable for the development of clinical tissue-engineered tubular organs? J Biomed Mater Res A. 2014; Jul. 102(7):2427–47.
Article17. Yamamoto Y, Nakamura T, Shimizu Y, Takimoto Y, Matsumoto K, Kiyotani T, et al. Experimental replacement of the thoracic esophagus with a bioabsorbable collagen sponge scaffold supported by a silicone stent in dogs. ASAIO J. 1999; Jul–Aug. 45(4):311–6.
Article18. Mallis P, Chachlaki P, Katsimpoulas M, Stavropoulos-Giokas C, Michalopoulos E. Optimization of decellularization procedure in rat esophagus for possible development of a tissue engineered construct. Bioengineering (Basel). 2018; Dec. 6(1):3.
Article19. Dua KS, Hogan WJ, Aadam AA, Gasparri M. In-vivo oesophageal regeneration in a human being by use of a non-biological scaffold and extracellular matrix. Lancet. 2016; Jul. 388(10039):55–61.
Article20. Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010; Nov. 31(31):7971–7.
Article21. Kim IG, Hwang MP, Du P, Ko J, Ha CW, Do SH, et al. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials. 2015; May. 50:75–86.
Article22. Kim IG, Ko J, Lee HR, Do SH, Park K. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials. 2016; Apr. 85:18–29.
Article23. Oshima T, Gedda K, Koseki J, Chen X, Husmark J, Watari J, et al. Establishment of esophageal-like non-keratinized stratified epithelium using normal human bronchial epithelial cells. Am J Physiol Cell Physiol. 2011; Jun. 300(6):C1422–9.
Article24. Paunescu V, Deak E, Herman D, Siska IR, Tanasie G, Bunu C, et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007; May–Jun. 11(3):502–8.
Article25. Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010; May. 137(9):1407–20.
Article26. Giroux V, Lento AA, Islam M, Pitarresi JR, Kharbanda A, Hamilton KE, et al. Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J Clin Invest. 2017; Jun. 127(6):2378–91.
Article27. Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012; Feb. 1(2):142–9.
Article28. Navas A, Magana-Guerrero FS, Dominguez-Lopez A, Chavez-Garcia C, Partido G, Graue-Hernandez EO, et al. Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl Med. 2018; Dec. 7(12):906–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
- The Effect of Polyurethane Scaffold Surface Treatments on the Adhesion of Chondrocytes Subjected to Interstitial Perfusion Culture
- From In Vitro to Perioperative Vascular Tissue Engineering: Shortening Production Time by Traceable Textile-Reinforcement
- Strategies for Constructing Tissue-Engineered Fat for Soft Tissue Regeneration
- Secondary Esophageal Reconstruction for Esophageal Atresia