Endocrinol Metab.
2023 Apr;38(2):260-268. 10.3803/EnM.2023.1663.
Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of General Internal Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Cheongju St. Mary’s Hospital, Cheongju, Korea
- KMID: 2541882
- DOI: http://doi.org/10.3803/EnM.2023.1663
Abstract
- Background
Persistence with denosumab in male patients has not been adequately investigated, although poor denosumab persistence is associated with a significant risk of rebound vertebral fractures.
Methods
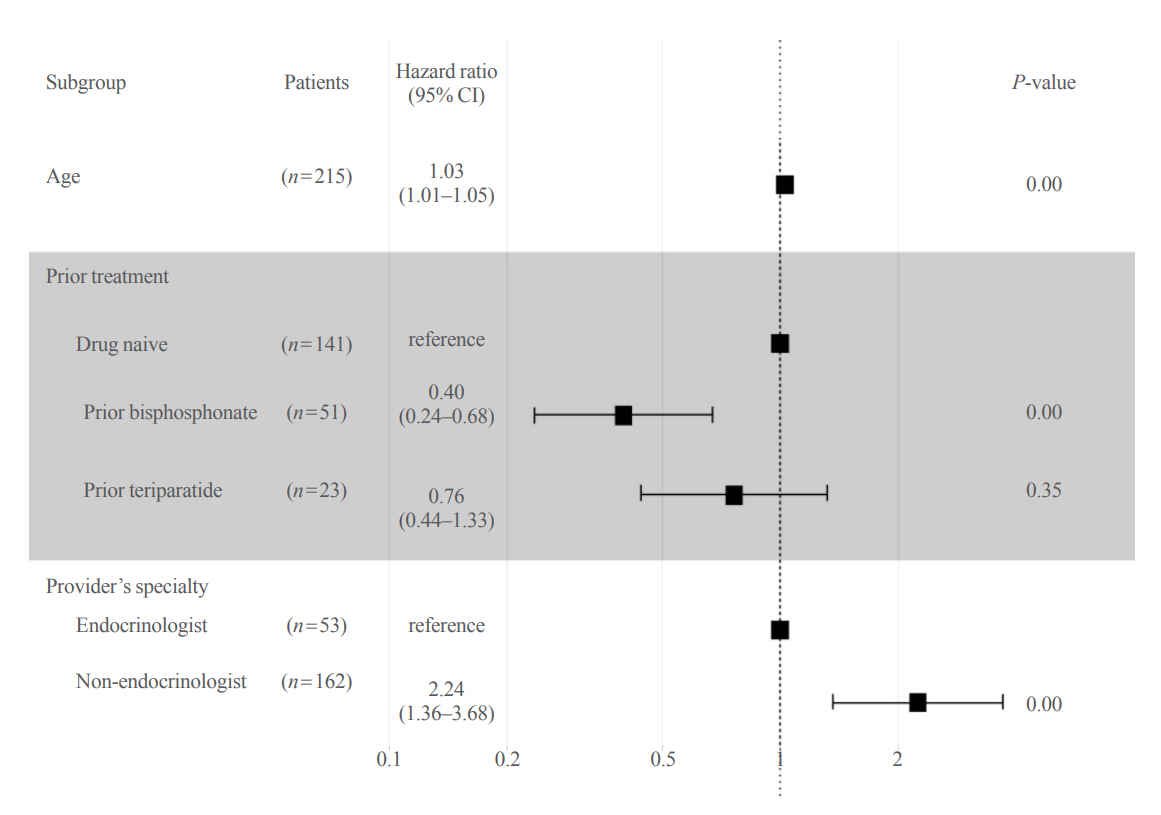
We retrospectively evaluated 294 Korean male osteoporosis patients treated with denosumab at three medical centers and examined their persistence with four doses of denosumab injection over 24 months of treatment. Persistence was defined as the extent to which a patient adhered to denosumab treatment in terms of the prescribed interval and dose, with a permissible gap of 8 weeks. For patients who missed their scheduled treatment appointment(s) during the follow-up period (i.e., no-shows), Cox proportional regression analysis was conducted to explore the factors associated with poor adherence. Several factors were considered, such as age, prior anti-osteoporotic drug use, the treatment provider’s medical specialty, the proximity to the medical center, and financial burdens of treatment.
Results
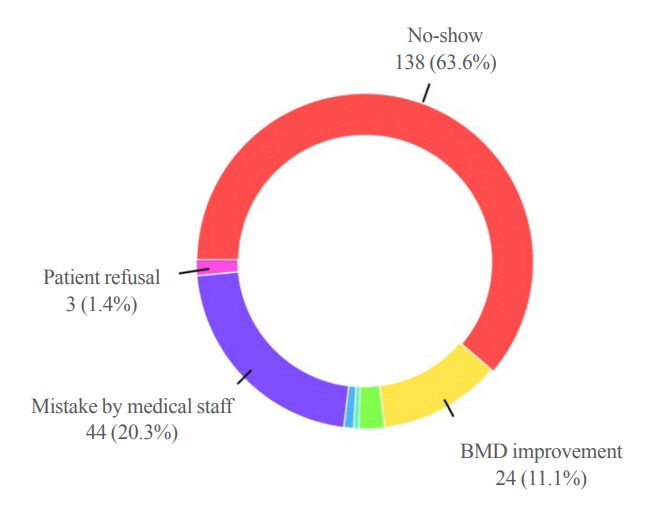
Out of 294 male patients, 77 (26.2%) completed all four sequential rounds of the denosumab treatment. Out of 217 patients who did not complete the denosumab treatment, 138 (63.6%) missed the scheduled treatment(s). Missing treatment was significantly associated with age (odds ratio [OR], 1.03), prior bisphosphonate use (OR, 0.76), and prescription by non-endocrinologists (OR, 2.24). Denosumab was stopped in 44 (20.3%) patients due to medical errors, in 24 (11.1%) patients due to a T-score improvement over –2.5, and in five (2.3%) patients due to expected dental procedures.
Conclusion
Our study showed that only one-fourth of Korean male osteoporosis patients were fully adherent to 24 months of denosumab treatment.
Keyword
Figure
Reference
-
1. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002; 359:1761–7.
Article2. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–65.
Article3. Rhee Y, Chang DG, Ha J, Kim S, Lee Y, Jo E, et al. Real-world safety and effectiveness of denosumab in patients with osteoporosis: a prospective, observational study in South Korea. Endocrinol Metab (Seoul). 2022; 37:497–505.
Article4. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018; 33:190–8.
Article5. Tay WL, Tay D. Discontinuing denosumab: can it be done safely?: a review of the literature. Endocrinol Metab (Seoul). 2022; 37:183–94.
Article6. Kim BK, Kim CH, Min YK. Preventing rebound-associated fractures after discontinuation of denosumab therapy: a position statement from the Health Insurance Committee of the Korean Endocrine Society. Endocrinol Metab (Seoul). 2021; 36:909–11.
Article7. Lyu H, Yoshida K, Zhao SS, Wei J, Zeng C, Tedeschi SK, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020; 173:516–26.8. Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and allcause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011; 17:25–39.
Article9. Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res. 2012; 27:202–10.
Article10. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007; 22:465–75.
Article11. Cho H, Byun JH, Song I, Kim HY, Ha YC, Kim TY, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018; 97:e11470.
Article12. Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int. 2010; 86:202–10.
Article13. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006; 17:1726–33.
Article14. Korean Society or Bone and Mineral Research. Osteoporosis and osteoporotic fracture fact sheet 2019 [Internet]. Seoul: Korean Society or Bone and Mineral Research;2019. [cited 2023 Apr 3]. Available from: https://www.ksbmr.org/bbs/skin/notice_popup_k/download.php?code=fact&number=1223.15. Haentjens P, Magaziner J, Colon-Emeric CS, Vander-schueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010; 152:380–90.
Article16. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008; 29:441–64.
Article17. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008; 11:44–7.
Article18. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012; 73:691–705.
Article19. Tremblay E, Perreault S, Dorais M. Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos. 2016; 11:30.
Article20. Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O. Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int. 2015; 26:2401–11.
Article21. Lakatos P, Takacs I, Marton I, Toth E, Zoltan C, Lang Z, et al. A retrospective longitudinal database study of persistence and compliance with treatment of osteoporosis in Hungary. Calcif Tissue Int. 2016; 98:215–25.
Article22. Boschitsch E, Naegele O, Klinger A, Brix-Samoylenko H. Long-term persistence with denosumab: real-world data from the Austrian Osteoporosis Clinic (AOC): a retrospective data analysis. Osteoporos Int. 2022; 33:263–72.
Article23. Borek DM, Smith RC, Gruber CN, Gruber BL. Long-term persistence in patients with osteoporosis receiving denosumab in routine practice: 36-month non-interventional, observational study. Osteoporos Int. 2019; 30:1455–64.
Article24. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009; 24:63–71.
Article25. Chandran M, Hao Y, Kwee AK, Cheen MH, Chin YA, Ng VY. Adherence to dosing schedule of denosumab therapy for osteoporosis during COVID-19 lockdown: an electronic medical record and pharmacy claims database study from Asia. Osteoporos Int. 2022; 33:251–61.
Article26. Cheng LI, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, et al. Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm. 2015; 21:824–33.
Article27. Fuksa L, Vytrisalova M. Adherence to denosumab in the treatment of osteoporosis and its utilization in the Czech Republic. Curr Med Res Opin. 2015; 31:1645–53.
Article28. Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos. 2017; 12:22.
Article29. Modi A, Sajjan S, Insinga R, Weaver J, Lewiecki EM, Harris ST. Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int. 2017; 28:1355–63.
Article30. Ban JK, Hao BB, McCarthy L, Guilcher SJ, Cadarette SM. Denosumab utilization among older adults in Ontario: patient characteristics, persistence with therapy, and return to therapy after an extended gap. Osteoporos Int. 2019; 30:1865–72.
Article31. Walsh ME, Fahey T, Moriarty F. Persistence with oral bisphosphonates and denosumab among older adults in primary care in Ireland. Arch Osteoporos. 2021; 16:71.
Article32. Morley J, Moayyeri A, Ali L, Taylor A, Feudjo-Tepie M, Hamilton L, et al. Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int. 2020; 31:533–45.
Article33. Yazan CD, Bugdayci O, Ilgin C, Yavuz DG. Effect of denosumab treatment on bone mineral density and bone turnover markers in osteoporotic patients: real-life experience 2-year follow-up. Arch Osteoporos. 2022; 17:125.
Article34. Hattori K, Takahashi N, Kojima T, Imagama S. Risk factors for denosumab discontinuation in patients with postmenopausal osteoporosis. Mod Rheumatol. 2022; Jul. 5. [Epub]. https://doi.org/10.1093/mr/roac070.
Article35. van Boven JF, de Boer PT, Postma MJ, Vegter S. Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab. 2013; 31:562–70.
Article36. Sagalla N, Lee R, Sloane R, Lyles K, Colon-Emeric C. Factors associated with adherence to osteoporosis medications among male veterans. JBMR Plus. 2021; 5:e10498.
Article37. Garcia-Sempere A, Hurtado I, Sanfelix-Genoves J, Rodriguez-Bernal CL, Gil Orozco R, Peiro S, et al. Primary and secondary non-adherence to osteoporotic medications after hip fracture in Spain. The PREV2FO population-based retrospective cohort study. Sci Rep. 2017; 7:11784.
Article38. Tan HC, Seng JJB, Low LL. Osteoporosis awareness among patients in Singapore (OASIS): a community hospital perspective. Arch Osteoporos. 2021; 16:151.39. Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int. 2015; 26:2479–89.
Article40. Wamala S, Merlo J, Bostrom G, Hogstedt C, Agren G. Socioeconomic disadvantage and primary non-adherence with medication in Sweden. Int J Qual Health Care. 2007; 19:134–40.
Article41. Goldman DP, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004; 291:2344–50.
Article42. Campisi G, Mauceri R, Bertoldo F, Fusco V, Bedogni A. A pragmatic window of opportunity to minimise the risk of MRONJ development in individuals with osteoporosis on denosumab therapy: a hypothesis. Head Face Med. 2021; 17:25.
Article43. Yeam CT, Chia S, Tan HC, Kwan YH, Fong W, Seng JJ. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int. 2018; 29:2623–37.
Article44. Landfeldt E, Strom O, Robbins S, Borgstrom F. Adherence to treatment of primary osteoporosis and its association to fractures: the Swedish Adherence Register Analysis (SARA). Osteoporos Int. 2012; 23:433–43.
Article45. Hansen C, Pedersen BD, Konradsen H, Abrahamsen B. Anti-osteoporotic therapy in Denmark: predictors and demographics of poor refill compliance and poor persistence. Osteoporos Int. 2013; 24:2079–97.
Article46. Yun H, Curtis JR, Guo L, Kilgore M, Muntner P, Saag K, et al. Patterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiaries. BMC Musculoskelet Disord. 2014; 15:112.
Article47. Devine J, Trice S, Finney Z, Yarger S, Nwokeji E, Linton A, et al. A retrospective analysis of extended-interval dosing and the impact on bisphosphonate compliance in the US Military Health System. Osteoporos Int. 2012; 23:1415–24.
Article48. Curtis JR, Xi J, Westfall AO, Cheng H, Lyles K, Saag KG, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009; 47:334–41.49. Kyvernitakis I, Kostev K, Kurth A, Albert US, Hadji P. Differences in persistency with teriparatide in patients with osteoporosis according to gender and health care provider. Osteoporos Int. 2014; 25:2721–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistence of Denosumab Therapy among Patients with Osteoporosis
- Denosumab for the treatment of osteoporosis
- Denosumab (RANKL Inhibitor): A Potent Anti-Resorptive Agent
- Long-Term Efficacy and Safety of Denosumab: Insights beyond 10 Years of Use
- Cost-Effectiveness of Denosumab for Post-Menopausal Osteoporosis in South Korea