Endocrinol Metab.
2023 Apr;38(2):203-213. 10.3803/EnM.2023.1673.
New Insights into Calorie Restriction Induced Bone Loss
- Affiliations
-
- 1MaineHealth Institute for Research, Scarborough, ME, USA
- KMID: 2541876
- DOI: http://doi.org/10.3803/EnM.2023.1673
Abstract
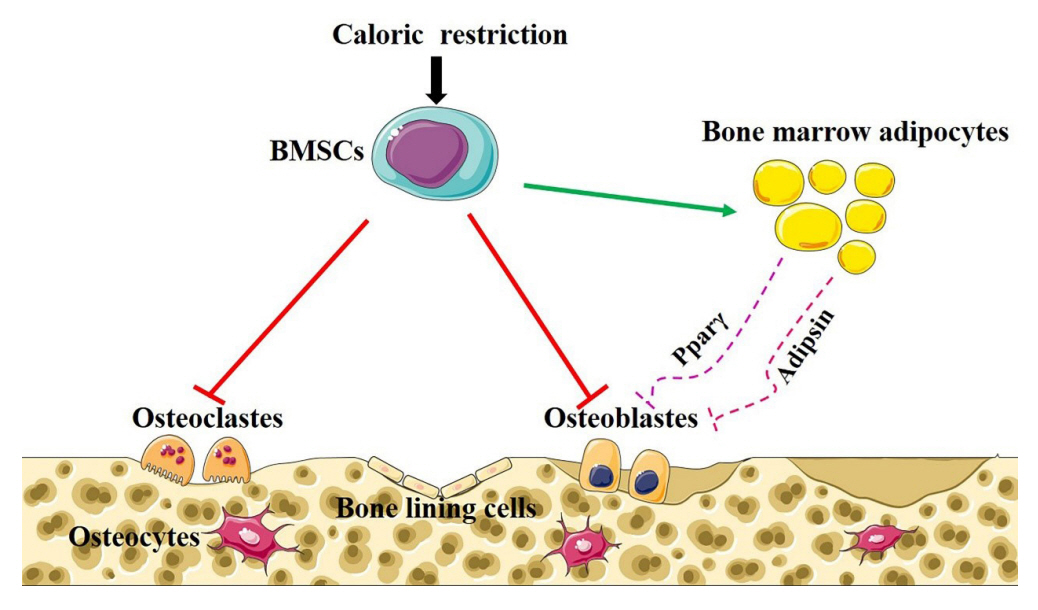
- Caloric restriction (CR) is now a popular lifestyle choice due to its ability in experimental animals to improve lifespan, reduce body weight, and lessen oxidative stress. However, more and more emerging evidence suggests this treatment requires careful consideration because of its detrimental effects on the skeletal system. Experimental and clinical studies show that CR can suppress bone growth and raise the risk of fracture, but the specific mechanisms are poorly understood. Reduced mechanical loading has long been thought to be the primary cause of weight loss-induced bone loss from calorie restriction. Despite fat loss in peripheral depots with calorie restriction, bone marrow adipose tissue (BMAT) increases, and this may play a significant role in this pathological process. Here, we update recent advances in our understanding of the effects of CR on the skeleton, the possible pathogenic role of BMAT in CR-induced bone loss, and some strategies to mitigate any potential side effects on the skeletal system.
Keyword
Figure
Reference
-
1. Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 2019; 29:592–610.2. Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017; 8:14063.3. Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018; 27:805–15.e4.4. Huang TH, Ables GP. Dietary restrictions, bone density, and bone quality. Ann N Y Acad Sci. 2016; 1363:26–39.5. Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006; 166:2502–10.6. Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008; 93:2181–7.7. Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010; 13:40–5.
Article8. Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012; 27:619–27.
Article9. Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res. 2016; 31:40–51.
Article10. Pachon-Pena G, Bredella MA. Bone marrow adipose tissue in metabolic health. Trends Endocrinol. Metab. 2022; 33:401–8.11. Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993; 95:131–40.
Article12. Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr. 1994; 48:561–6.13. Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011; 26:1339–48.
Article14. Bleicher K, Cumming RG, Naganathan V, Travison TG, Sambrook PN, Blyth FM, et al. The role of fat and lean mass in bone loss in older men: findings from the CHAMP study. Bone. 2011; 49:1299–305.15. Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, et al. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr. 2007; 85:972–80.
Article16. Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E, et al. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008; 168:1859–66.
Article17. Villalon KL, Gozansky WS, Van Pelt RE, Wolfe P, Jankowski CM, Schwartz RS, et al. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring). 2011; 19:2345–50.
Article18. Frolich J, Hansen S, Winkler LA, Andresen AK, Hermann AP, Stoving RK. The role of body weight on bone in anorexia nervosa: a HR-pQCT study. Calcif Tissue Int. 2017; 101:24–33.
Article19. Fazeli PK, Klibanski A. Effects of anorexia nervosa on bone metabolism. Endocr Rev. 2018; 39:895–910.
Article20. Liu CT, Sahni S, Xu H, McLean RR, Broe KE, Hannan MT, et al. Long-term and recent weight change are associated with reduced peripheral bone density, deficits in bone microarchitecture, and decreased bone strength: the Framingham Osteoporosis Study. J Bone Miner Res. 2018; 33:1851–8.
Article21. LaMothe JM, Hepple RT, Zernicke RF. Selected contribution: bone adaptation with aging and long-term caloric restriction in Fischer 344 x Brown-Norway F1-hybrid rats. J Appl Physiol (1985). 2003; 95:1739–45.22. Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One. 2012; 7:e51357.
Article23. Cao JJ. Caloric restriction combined with exercise is effective in reducing adiposity and mitigating bone structural deterioration in obese rats. Ann N Y Acad Sci. 2018; 1433:41–52.
Article24. Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010; 25:2078–88.
Article25. Devlin MJ, Brooks DJ, Conlon C, van Vliet M, Louis L, Rosen CJ, et al. Daily leptin blunts marrow fat but does not impact bone mass in calorie-restricted mice. J Endocrinol. 2016; 229:295–306.
Article26. Maridas DE, Rendina-Ruedy E, Helderman RC, DeMambro VE, Brooks D, Guntur AR, et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. FASEB J. 2019; 33:2885–98.
Article27. Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med. 2006; 354:2250–61.28. Villareal DT, Kotyk JJ, Armamento-Villareal RC, Kenguva V, Seaman P, Shahar A, et al. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell. 2011; 10:96–102.
Article29. Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res. 2010; 25:527–36.
Article30. Premaor MO, Compston JE, Fina Aviles F, Pages-Castella A, Nogues X, Diez-Perez A, et al. The association between fracture site and obesity in men: a population-based cohort study. J Bone Miner Res. 2013; 28:1771–7.
Article31. Choi YJ, Kim DJ, Lee Y, Chung YS. Insulin is inversely associated with bone mass, especially in the insulin-resistant population: the Korea and US National Health and Nutrition Examination Surveys. J Clin Endocrinol Metab. 2014; 99:1433–41.
Article32. Cauley JA, Burghardt AJ, Harrison SL, Cawthon PM, Schwartz AV, Connor EB, et al. Accelerated bone loss in older men: effects on bone microarchitecture and strength. J Bone Miner Res. 2018; 33:1859–69.
Article33. Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013; 98:1923–9.
Article34. Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, et al. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008; 249:938–46.
Article35. Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL, et al. Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr. 2015; 101:659–67.
Article36. Behrendt AK, Kuhla A, Osterberg A, Polley C, Herlyn P, Fischer DC, et al. Dietary restriction-induced alterations in bone phenotype: effects of lifelong versus short-term caloric restriction on femoral and vertebral bone in C57BL/6 mice. J Bone Miner Res. 2016; 31:852–63.
Article37. Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008; 23:870–8.
Article38. Shen CL, Zhu W, Gao W, Wang S, Chen L, Chyu MC. Energy-restricted diet benefits body composition but degrades bone integrity in middle-aged obese female rats. Nutr Res. 2013; 33:668–76.
Article39. Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987; 219:1–9.
Article40. Frost HM. Obesity, and bone strength and “mass”: a tutorial based on insights from a new paradigm. Bone. 1997; 21:211–4.
Article41. Ensrud KE, Lipschutz RC, Cauley JA, Seeley D, Nevitt MC, Scott J, et al. Body size and hip fracture risk in older women: a prospective study. Am J Med. 1997; 103:274–80.42. Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. J Bone Miner Res. 1999; 14:1622–7.
Article43. Hyldstrup L, Andersen T, McNair P, Breum L, Transbol I. Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol (Copenh). 1993; 129:393–8.
Article44. Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001; 12:199–206.
Article45. Nandy A, Rendina-Ruedy E. Bone marrow adipocytes: good, bad, or just different? Best Pract Res Clin Endocrinol Metab. 2021; 35:101550.46. Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014; 508:269–73.
Article47. Deng P, Yuan Q, Cheng Y, Li J, Liu Z, Liu Y, et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell. 2021; 28:1057–73.e7.
Article48. Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone. 2017; 97:38–42.
Article49. Bredella MA, Gill CM, Gerweck AV, Landa MG, Kumar V, Daley SM, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013; 269:534–41.
Article50. Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013; 28:1721–8.
Article51. Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017; 95:85–90.
Article52. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014; 20:368–75.
Article53. Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007; 21:1580–4.
Article54. Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015; 6:7808.
Article55. Aaron N, Kraakman MJ, Zhou Q, Liu Q, Costa S, Yang J, et al. Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells. Elife. 2021; 10:e69209.
Article56. Li Z, Bagchi DP, Zhu J, Bowers E, Yu H, Hardij J, et al. Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight. 2022; 7:e160915.
Article57. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006; 29:1337–44.58. Duarte FO, Sene-Fiorese M, Manzoni MS, de Freitas LF, Cheik NC, Garcia de Oliveira Duarte AC, et al. Caloric restriction and refeeding promoted different metabolic effects in fat depots and impaired dyslipidemic profile in rats. Nutrition. 2008; 24:177–86.
Article59. Maio MT, Hannan JL, Komolova M, Adams MA. Caloric restriction prevents visceral adipose tissue accumulation and maintains erectile function in aging rats. J Sex Med. 2012; 9:2273–83.
Article60. Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006; 61:943–50.
Article61. Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol (1985). 2012; 112:79–85.
Article62. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009; 94:2129–36.
Article63. Bredella MA, Karzar NH, Singhal V, Bose A, Animashaun A, Mitchell DM, et al. Impact of sleeve gastrectomy on bone outcomes in adolescents vs. adults with obesity. Bone. 2021; 149:115975.
Article64. Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010; 25:298–304.
Article65. Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012; 27:1864–71.
Article66. Fazeli PK, Bredella MA, Pachon-Pena G, Zhao W, Zhang X, Faje AT, et al. The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight. 2021; 6:e138636.
Article67. Bredella MA, Singhal V, Hazhir Karzar N, Animashaun A, Bose A, Stanford FC, et al. Effects of sleeve gastrectomy on bone marrow adipose tissue in adolescents and young adults with obesity. J Clin Endocrinol Metab. 2020; 105:e3961–70.
Article68. Blom-Hogestol IK, Mala T, Kristinsson JA, Hauge EM, Brunborg C, Gulseth HL, et al. Changes in bone marrow adipose tissue one year after Roux-en-Y Gastric bypass: a prospective cohort study. J Bone Miner Res. 2019; 34:1815–23.
Article69. Tencerova M, Kassem M. The bone marrow-derived stromal cells: commitment and regulation of adipogenesis. Front Endocrinol (Lausanne). 2016; 7:127.
Article70. Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007; 13:1496–503.71. Khan E, Abu-Amer Y. Activation of peroxisome proliferator-activated receptor-gamma inhibits differentiation of preosteoblasts. J Lab Clin Med. 2003; 142:29–34.
Article72. Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999; 74:357–71.73. Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016; 23:1128–39.
Article74. Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, et al. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989; 244:1483–7.
Article75. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–56.
Article76. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20:345–57.
Article77. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011; 17:1235–41.
Article78. Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017; 25:661–72.
Article79. Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z, et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Invest. 2021; 131:e140214.
Article80. Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H, et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021; 22:e52481.
Article81. Attane C, Esteve D, Chaoui K, Iacovoni JS, Corre J, Moutahir M, et al. Human bone marrow is comprised of adipocytes with specific lipid metabolism. Cell Rep. 2020; 30:949–58.e6.
Article82. Li Z, Bowers E, Zhu J, Yu H, Hardij J, Bagchi DP, et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. Elife. 2022; 11:e78496.
Article83. Shimizu E, Selvamurugan N, Westendorf JJ, Partridge NC. Parathyroid hormone regulates histone deacetylases in osteoblasts. Ann N Y Acad Sci. 2007; 1116:349–53.
Article84. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001; 344:1434–41.
Article85. Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res. 2003; 18:18–23.
Article86. Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995; 136:3632–8.
Article87. Lotinun S, Sibonga JD, Turner RT. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002; 17:29–36.
Article88. Esen E, Lee SY, Wice BM, Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res. 2015; 30:1959–68.
Article89. Ben-awadh AN, Delgado-Calle J, Tu X, Kuhlenschmidt K, Allen MR, Plotkin LI, et al. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology. 2014; 155:2797–809.
Article90. Delgado-Calle J, Hancock B, Likine EF, Sato AY, McAndrews K, Sanudo C, et al. MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production. FASEB J. 2018; 32:2878–90.
Article91. Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011; 49:636–43.
Article92. Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010; 21:237–44.
Article93. Wang J, Wang X, Holz JD, Rutkowski T, Wang Y, Zhu Z, et al. Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS One. 2013; 8:e74255.
Article94. Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012; 27:2001–14.
Article95. Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by downregulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology. 2001; 142:4900–9.
Article96. Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001; 107:277–86.
Article97. Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003; 88:695–705.
Article98. Feldman D, Wesley Pike J, Adams JS. Vitamin D. 3rd ed. London: Elsevier;2011. Chapter 61, Vitamin D and osteoporosis. p. 1129–44.99. Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D (3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J Bone Miner Res. 2011; 26:1145–53.
Article100. Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007; 158:1–235.101. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009; 339:b3692.102. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997; 94:9831–5.103. Piek E, Sleumer LS, van Someren EP, Heuver L, de Haan JR, de Grijs I, et al. Osteo-transcriptomics of human mesenchymal stem cells: accelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone. 2010; 46:613–27.
Article104. Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999; 65:173–80.105. Suda T, Ueno Y, Fujii K, Shinki T. Vitamin D and bone. J Cell Biochem. 2003; 88:259–66.
Article106. Kelly KA, Gimble JM. 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology. 1998; 139:2622–8.
Article107. Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2). Exp Gerontol. 2004; 39:333–8.108. Ding J, Nagai K, Woo JT. Insulin-dependent adipogenesis in stromal ST2 cells derived from murine bone marrow. Biosci Biotechnol Biochem. 2003; 67:314–21.
Article109. Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Sherrell RM, Field MP, et al. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr. 2013; 97:637–45.
Article110. Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011; 26:2851–9.
Article111. Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos Int. 2013; 24:2749–62.
Article112. Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int. 2015; 26:1605–18.
Article113. Hosny IA, Elghawabi HS, Younan WB, Sabbour AA, Gobrial MA. Beneficial impact of aerobic exercises on bone mineral density in obese premenopausal women under caloric restriction. Skeletal Radiol. 2012; 41:423–7.
Article114. Beavers KM, Beavers DP, Martin SB, Marsh AP, Lyles MF, Lenchik L, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2017; 72:1582–5.
Article115. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017; 376:1943–55.
Article116. Hell RC, Ocarino NM, Boeloni JN, Silva JF, Goes AM, Santos RL, et al. Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrow-derived mesenchymal stem cells. Acta Physiol (Oxf). 2012; 205:292–301.
Article117. Menuki K, Mori T, Sakai A, Sakuma M, Okimoto N, Shimizu Y, et al. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone. 2008; 43:613–20.
Article118. Bu S, Chen Y, Wang S, Zhang F, Ji G. Treadmill training regulates β-catenin signaling through phosphorylation of GSK-3β in lumbar vertebrae of ovariectomized rats. Eur J Appl Physiol. 2012; 112:3295–304.
Article119. Swift JM, Swift SN, Nilsson MI, Hogan HA, Bouse SD, Bloomfield SA. Cancellous bone formation response to simulated resistance training during disuse is blunted by concurrent alendronate treatment. J Bone Miner Res. 2011; 26:2140–50.
Article120. Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell. 2018; 175:1756–68. e17.
Article121. Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise regulation of marrow fat in the setting of PPARγ agonist treatment in female C57BL/6 mice. Endocrinology. 2015; 156:2753–61.
Article122. Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise decreases marrow adipose tissue through ß-oxidation in obese running mice. J Bone Miner Res. 2017; 32:1692–702.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Links between Caloric Restriction and Sir2/SIRT1 Activation
- The Effect of Detraining on Lipoprotein and Body Composition after 8 Week Calorie Restriction and Different Level of Aerobic Exercise among Obese Middle-aged Women
- Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy
- Mitochondrial DNA A3243G mutation in noise-induced sensorineural hearing loss
- The effects of weight loss by a low-calorie diet and a low-calorie plus exercise in overweight undergraduate students