Nutr Res Pract.
2023 Apr;17(2):371-385. 10.4162/nrp.2023.17.2.371.
Role of soy lecithin combined with soy isoflavone on cerebral blood flow in rats of cognitive impairment and the primary screening of its optimum combination
- Affiliations
-
- 1Department of Nutrition and Food Hygiene, School of Public Health, Capital Medical University, Beijing 100069, China
- 2Cadre Department, Beijing Jishuitan Hospital, Beijing 100035, China
- 3Medical Department, Beijing Shunyi Maternal and Child Health Hospital, Beijing 101300, China
- KMID: 2541372
- DOI: http://doi.org/10.4162/nrp.2023.17.2.371
Abstract
- BACKGROUND/OBJECTIVES
Soy isoflavone (SIF) and soy lecithin (SL) have beneficial effects on many chronic diseases, including neurodegenerative diseases. Regretfully, there is little evidence to show the combined effects of these soy extractives on the impairment of cognition and abnormal cerebral blood flow (CBF). This study examined the optimal combination dose of SIF + SL to provide evidence for improving CBF and protecting cerebrovascular endothelial cells.
MATERIALS/METHODS
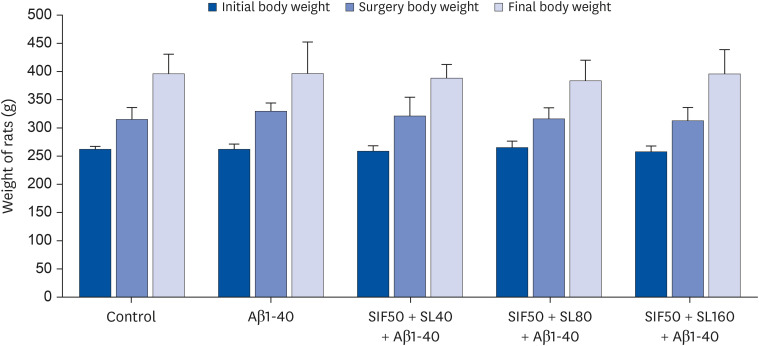
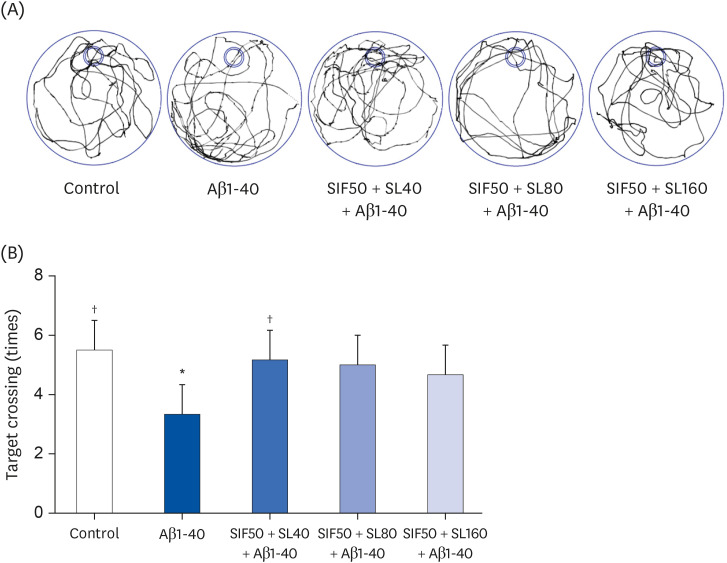
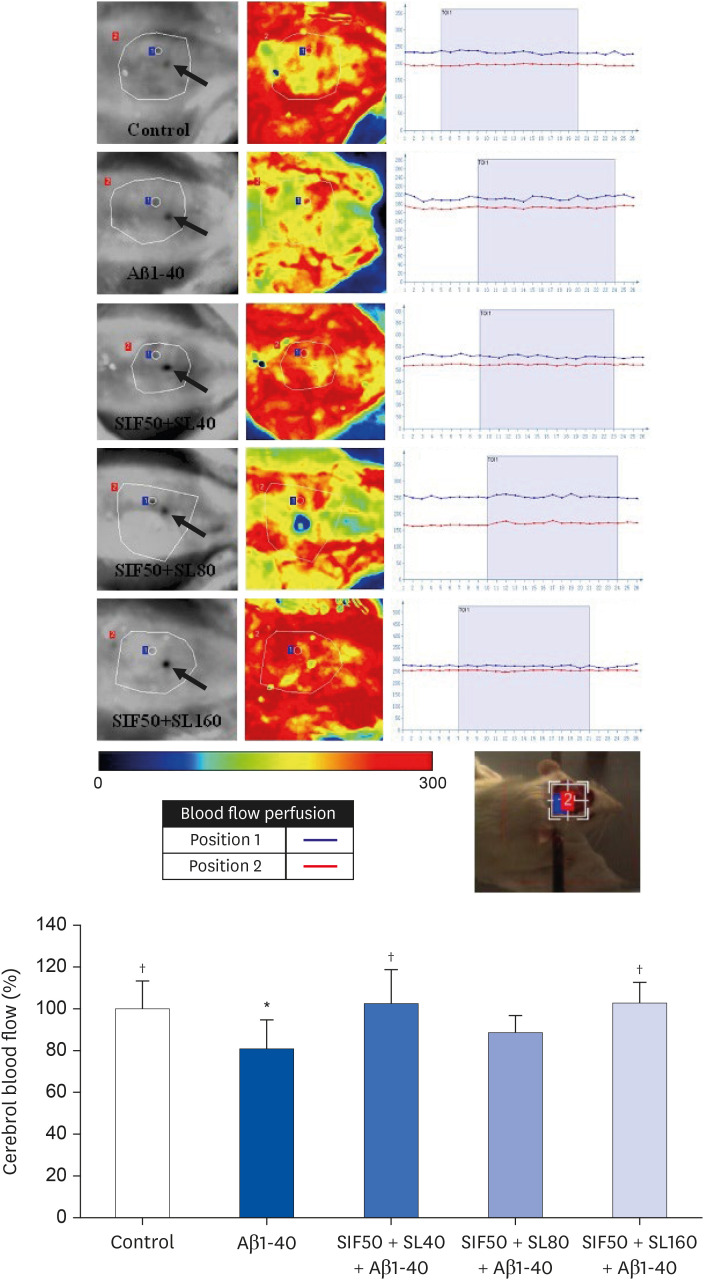
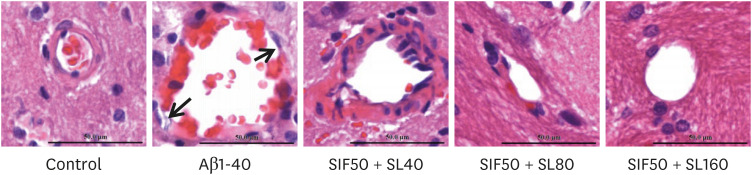
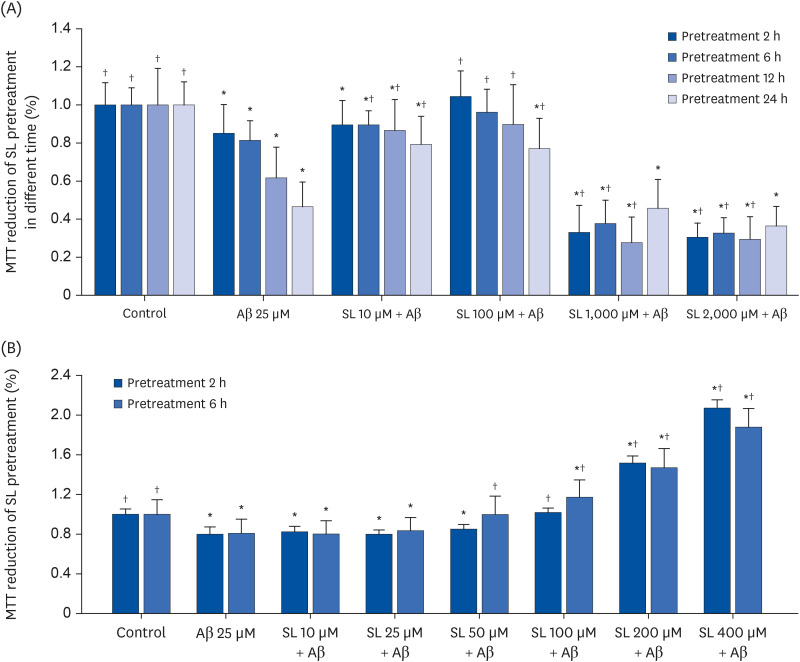
In vivo study, SIF50 + SL40, SIF50 + SL80 and SIF50 + SL160 groups were obtained. Morris water maze, laser speckle contrast imaging (LSCI), and hematoxylineosin staining were used to detect learning and memory impairment, CBF, and damage to the cerebrovascular tissue in rat. The 8-hydroxy-2′-deoxyguanosine (8-OHdG) and the oxidized glutathione (GSSG) were detected. The anti-oxidative damage index of superoxide dismutase (SOD) and glutathione (GSH) in the serum of an animal model was also tested. In vitro study, an immortalized mouse brain endothelial cell line (bEND.3 cells) was used to confirm the cerebrovascular endothelial cell protection of SIF + SL. In this study, 50 μM of Gen were used, while the 25, 50, or 100 μM of SL for different incubation times were selected first. The intracellular levels of 8-OHdG, SOD, GSH, and GSSG were also detected in the cells.
RESULTS
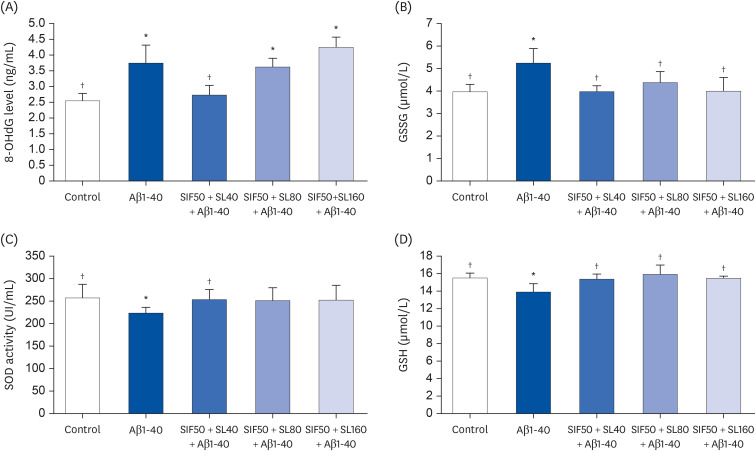
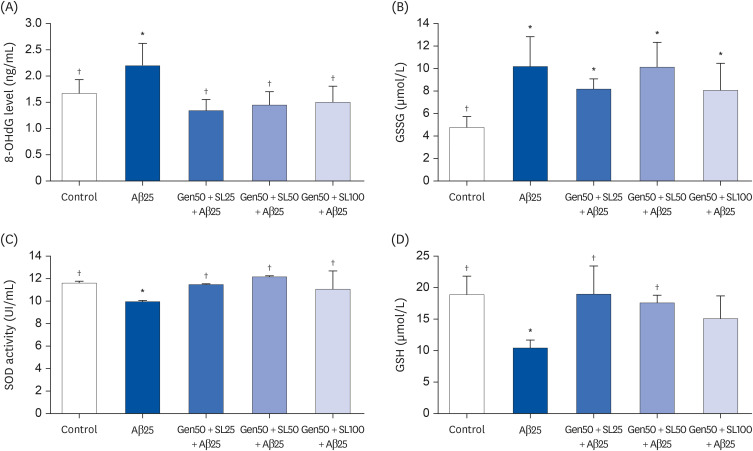
In vivo study, SIF + SL could increase the target crossing times significantly and shorten the total swimming distance of rats. The CBF in the rats of the SIF50 + SL40 group and SIF50 + SL160 group was enhanced. Pathological changes, such as attenuation of the endothelium in cerebral vessels were much less in the SIF50 + SL40 group and SIF50 + SL160 group. The 8-OHdG was reduced in the SIF50 + SL40 group. The GSSG showed a significant decrease in all SIF + SL pretreatment groups, but the GSH showed an opposite result. SOD was upregulated by SIF + SL pretreatment. Different combinations of Genistein (Gen)+SL, the secondary proof of health benefits found in vivo study, showed they have effective antioxidation and less side reaction on protecting cerebrovascular endothelial cell. SIF50 + SL40 in rats experiment and Gen50 + SL25 in cell test were the optimum joint doses on alleviating cognitive impairment and regulating CBF through protecting cerebrovascular tissue by its antioxidant activity.
CONCLUSIONS
SIF+SL could significantly prevent cognitive defect induced by β-Amyloid through regulating CBF. This kind of effect might be attributed to its antioxidant activity on protecting cerebral vessels.
Figure
Reference
-
1. Wu HZ, Ong KL, Seeher K, Armstrong NJ, Thalamuthu A, Brodaty H, Sachdev P, Mather K. Circulating microRNAs as biomarkers of Alzheimer’s disease: a systematic review. J Alzheimers Dis. 2016; 49:755–766. PMID: 26484928.2. Rius-Pérez S, Tormos AM, Pérez S, Taléns-Visconti R. Vascular pathology: cause or effect in Alzheimer disease? Neurologia (Engl Ed). 2018; 33:112–120. PMID: 26385017.3. Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S, Belden C, Maarouf CL, Thiyyagura P, Mo H, et al. Cerebral blood flow in Alzheimer’s disease. Vasc Health Risk Manag. 2012; 8:599–611. PMID: 23109807.4. Xi YD, Yu HL, Ding J, Ma WW, Yuan LH, Feng JF, Xiao YX, Xiao R. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr Neurovasc Res. 2012; 9:32–41. PMID: 22272764.5. Soybean IS. Soybean and processed soy foods ingredients, and their role in cardiometabolic risk prevention. Recent Pat Food Nutr Agric. 2015; 7:75–82. PMID: 26118770.6. Mourad AM, de Carvalho Pincinato E, Mazzola PG, Sabha M, Moriel P. Influence of soy lecithin administration on hypercholesterolemia. Cholesterol. 2010; 2010:824813. PMID: 21490917.7. Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017; 9:324. PMID: 28338639.8. Xi YD, Yu HL, Ma WW, Ding BJ, Ding J, Yuan LH, Feng JF, Xiao R. Genistein inhibits mitochondrial-targeted oxidative damage induced by β-amyloid peptide 25-35 in PC12 cells. J Bioenerg Biomembr. 2011; 43:399–407. PMID: 21732176.9. Ding BJ, Ma WW, He LL, Zhou X, Yuan LH, Yu HL, Feng JF, Xiao R. Soybean isoflavone alleviates β-amyloid 1-42 induced inflammatory response to improve learning and memory ability by down regulation of Toll-like receptor 4 expression and nuclear factor-κB activity in rats. Int J Dev Neurosci. 2011; 29:537–542. PMID: 21515354.10. Xi YD, Li XY, Yu HL, Jing H, Ma WW, Yuan LH, Zhang DD, Wu J, Xiao R. Soy isoflavone antagonizes the oxidative cerebrovascular injury induced by β-amyloid peptides 1-42 in rats. Neurochem Res. 2014; 39:1374–1381. PMID: 24810766.11. Baumgartner S, Bruckert E, Gallo A, Plat J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis. 2020; 311:116–123. PMID: 32861515.12. Evans M, Njike VY, Hoxley M, Pearson M, Katz DL. Effect of soy isoflavone protein and soy lecithin on endothelial function in healthy postmenopausal women. Menopause. 2007; 14:141–149. PMID: 17006376.13. Xi YD, Li XY, Ding J, Yu HL, Ma WW, Yuan LH, Wu J, Xiao R. Soy isoflavone alleviates Aβ1-42-induced impairment of learning and memory ability through the regulation of RAGE/LRP-1 in neuronal and vascular tissue. Curr Neurovasc Res. 2013; 10:144–156. PMID: 23469956.14. Dunn AK. Laser speckle contrast imaging of cerebral blood flow. Ann Biomed Eng. 2012; 40:367–377. PMID: 22109805.15. Miners JS, Palmer JC, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer’s disease. Brain Pathol. 2016; 26:533–541. PMID: 26452729.16. Goulay R, Mena Romo L, Hol EM, Dijkhuizen RM. From stroke to dementia: a comprehensive review exposing tight interactions between stroke and amyloid-β formation. Transl Stroke Res. 2020; 11:601–614. PMID: 31776837.17. Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease--a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis. 2015; 82:593–606. PMID: 26311408.18. Xu J, Hu H, Chen B, Yue R, Zhou Z, Liu Y, Zhang S, Xu L, Wang H, Yu Z. Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS One. 2015; 10:e0136443. PMID: 26291709.19. Ma W, Ding B, Yu H, Yuan L, Xi Y, Xiao R. Genistein alleviates β-amyloid-induced inflammatory damage through regulating Toll-like receptor 4/nuclear factor κB. J Med Food. 2015; 18:273–279. PMID: 25384233.20. Ding J, Yu HL, Ma WW, Xi YD, Zhao X, Yuan LH, Feng JF, Xiao R. Soy isoflavone attenuates brain mitochondrial oxidative stress induced by β-amyloid peptides 1-42 injection in lateral cerebral ventricle. J Neurosci Res. 2013; 91:562–567. PMID: 23239252.21. Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging (Albany NY). 2012; 4:590–605. PMID: 23001356.22. Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J Clin Biochem Nutr. 2010; 47:246–255. PMID: 21103034.23. Punithavathi VR, Prince PS. Combined effects of quercetin and alpha-tocopherol on lipids and glycoprotein components in isoproterenol induced myocardial infarcted Wistar rats. Chem Biol Interact. 2009; 181:322–327. PMID: 19595682.24. Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020; 140:793–810. PMID: 32865691.25. Guo Y, Li X, Zhang M, Chen N, Wu S, Lei J, Wang Z, Wang R, Wang J, Liu H. Age- and brain region-associated alterations of cerebral blood flow in early Alzheimer’s disease assessed in AβPPSWE/PS1ΔE9 transgenic mice using arterial spin labeling. Mol Med Rep. 2019; 19:3045–3052. PMID: 30816468.26. Qiu L, Ng G, Tan EK, Liao P, Kandiah N, Zeng L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci Rep. 2016; 6:23964. PMID: 27050297.27. Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011; 26(Suppl 3):123–133. PMID: 21971457.28. Aabdallah DM, Eid NI. Possible neuroprotective effects of lecithin and alpha-tocopherol alone or in combination against ischemia/reperfusion insult in rat brain. J Biochem Mol Toxicol. 2004; 18:273–278. PMID: 15549708.29. Zhao H, Liang J, Li X, Yu H, Li X, Xiao R. Folic acid and soybean isoflavone combined supplementation protects the post-neural tube closure defects of rodents induced by cyclophosphamide in vivo and in vitro . Neurotoxicology. 2010; 31:180–187. PMID: 20060418.30. Sadhukhan P, Saha S, Dutta S, Mahalanobish S, Sil PC. Nutraceuticals: an emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol Res. 2018; 129:100–114. PMID: 29183770.31. Das SK, Gupta G, Rao DN, Vasudevan DM. Effect of lecithin with vitamin-B complex and tocopheryl acetate on long-term effect of ethanol induced immunomodulatory activities. Indian J Exp Biol. 2007; 45:683–688. PMID: 17877144.32. Kim SB, Assefa F, Lee SJ, Park EK, Kim SS. Combined effects of soy isoflavone and lecithin on bone loss in ovariectomized mice. Nutr Res Pract. 2021; 15:541–554. PMID: 34603603.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined effects of soy isoflavone and lecithin on bone loss in ovariectomized mice
- Validation of soy isoflavone intake and its health effects: a review of the development of exposure biomarkers
- Effect of Soy Isoflavone Intake on Water Maze Performance and Brain Acetylcholinesterase Activity in Rats
- Bioavailability Assessment of Isoflavones between Soybean and Soybean Sprout in Rat
- Effects of Soy Isoflavone Intake on Nitrite Content and Antioxidant Enzyme Activities in Male Rats Fed High-Fat Diet