Cancer Res Treat.
2023 Apr;55(2):636-642. 10.4143/crt.2022.343.
Neoadjuvant Nivolumab Plus Gemcitabine/Cisplatin Chemotherapy in Muscle-Invasive Urothelial Carcinoma of the Bladder
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Hematology-Oncology, Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea
- 3Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Radiology and Center for Imaging Sciences, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2541250
- DOI: http://doi.org/10.4143/crt.2022.343
Abstract
- Purpose
The activity and safety of neoadjuvant nivolumab plus gemcitabine/cisplatin (N+GC) were tested in patients with muscle-invasive bladder urothelial carcinoma (MIBC).
Materials and Methods
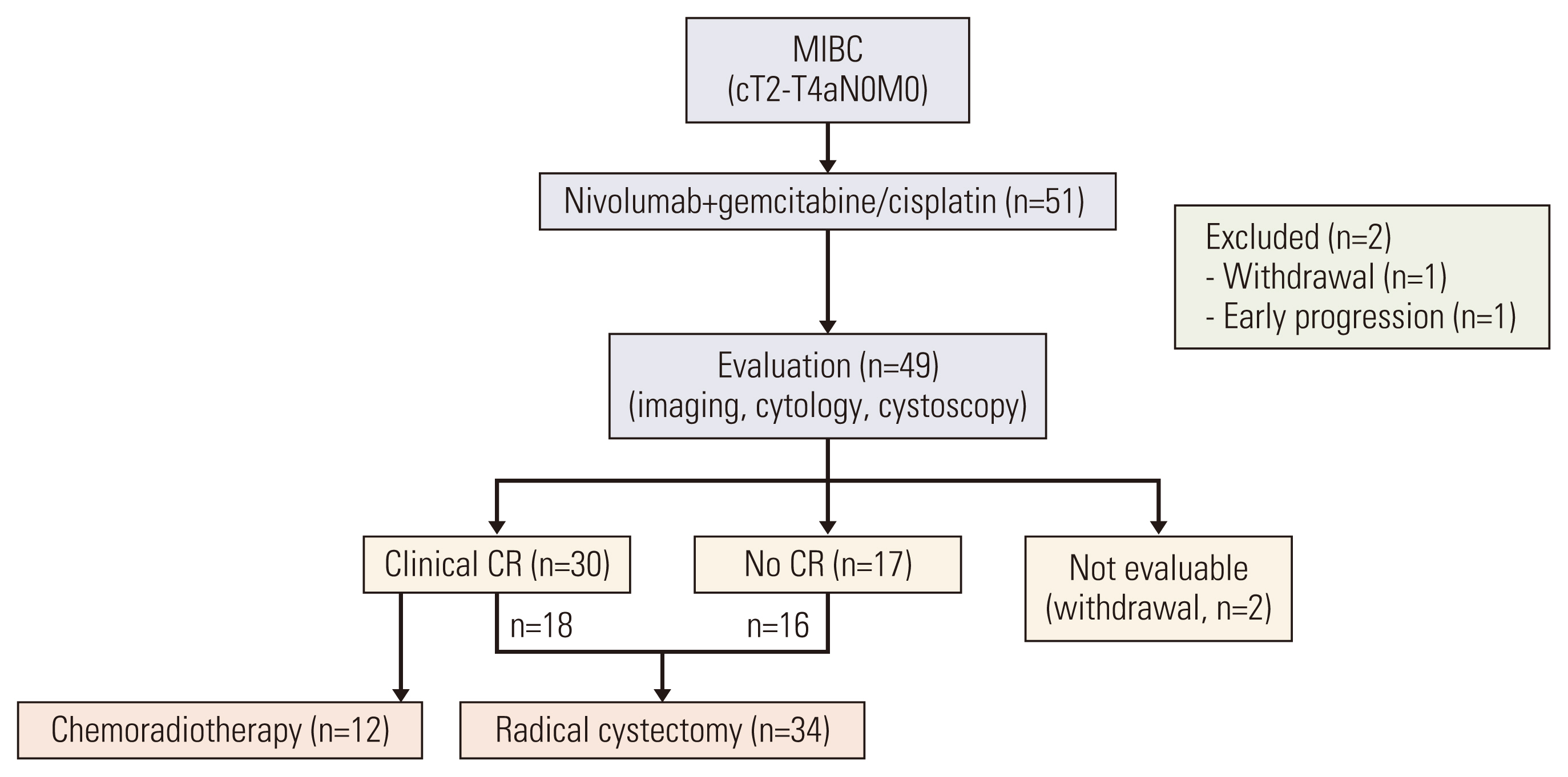
In a prospective phase II trial, patients with cT2-T4a N0 MIBC who were eligible for cisplatin and medically appropriate to undergo radical cystectomy (RC) were enrolled. Treatment with nivolumab 3 mg/kg on days 1 and 15 plus GC (cisplatin 70 mg/m2 on day 1, and gemcitabine 1,000 mg/m2 on days 1, 8, and 15) was repeated every 28 days up to 3 or 4 cycles, depending on the surgery schedules. The primary endpoint was pathologic complete response (pCR, ypT0). Secondary endpoints included pathologic downstaging (≤ ypT1), disease-free survival (DFS), and safety.
Results
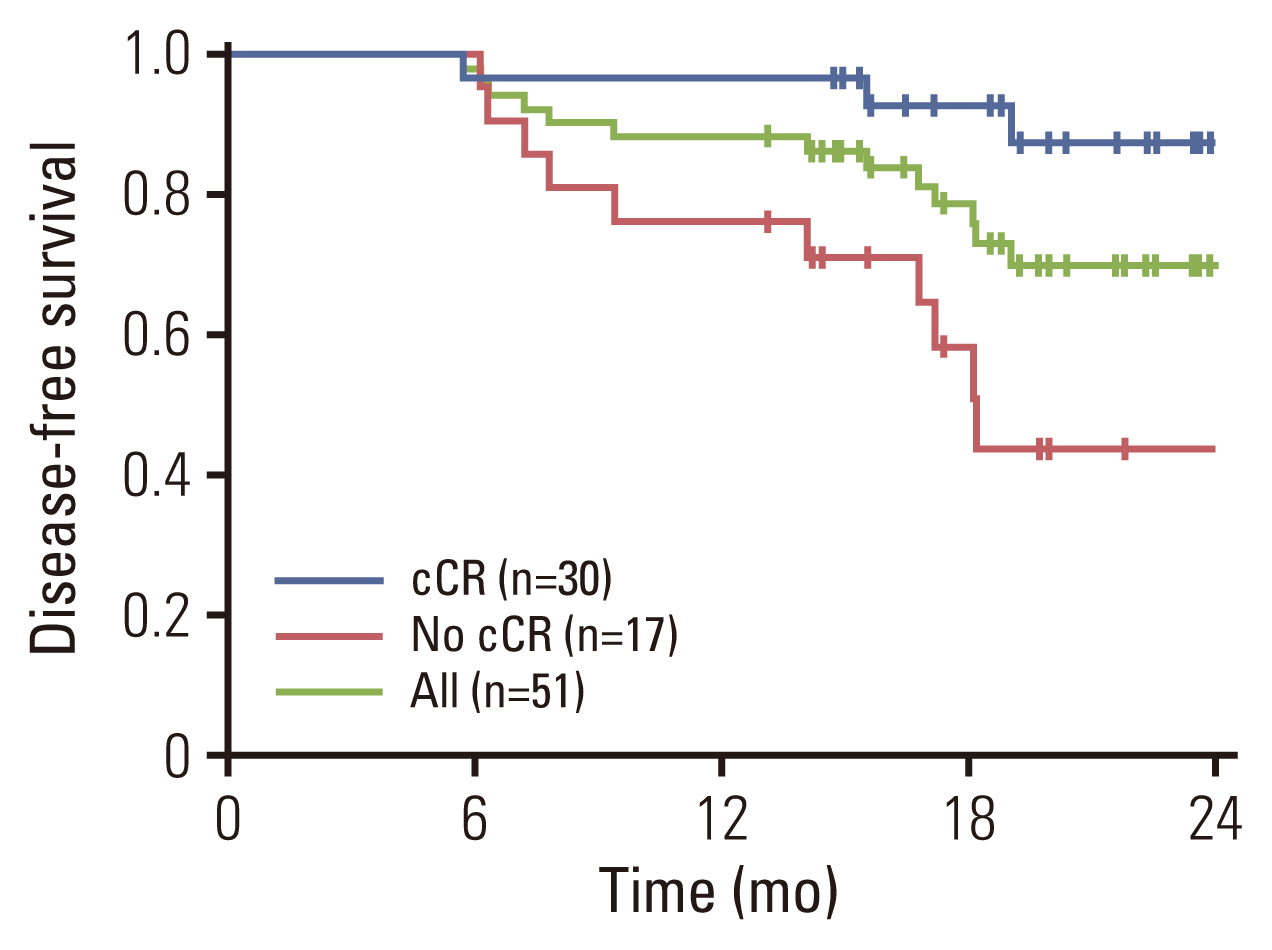
Between September 2019 and October 2020, 51 patients were enrolled. Neoadjuvant N+GC was well tolerated. Among 49 patients who completed neoadjuvant N+GC, clinical complete response (cCR) was achieved in 59% of intent-to-treat (ITT) population. RC was performed in 34 (69%) patients. pCR was achieved in 24% (12/49) of ITT population and 35% (12/34) of RC patients. Median DFS was not reached. Over a median follow-up of 24 months, 12 patients experienced disease recurrence and were treated with palliative therapy or surgery. Although 12 patients declined surgery and were treated with concurrent chemoradiotherapy, DFS was longer in patients with cCR after neoadjuvant therapy than those without. Preoperative programmed death-ligand 1 (PD-L1) did not correlate with pCR or pathologic downstaging rates.
Conclusion
Neoadjuvant N+GC was feasible and provided meaningful pathologic responses in patients with MIBC, regardless of baseline PD-L1 expression (ONO-4538-X41; CRIS.nih.go.kr, KCT0003804).
Keyword
Figure
Cited by 1 articles
-
Neoadjuvant Cisplatin-Based Chemotherapy Followed by Selective Bladder Preservation Chemoradiotherapy in Muscle-Invasive Urothelial Carcinoma of the Bladder:
Post Hoc Analysis of Two Prospective Studies
Sung Wook Cho, Sung Hee Lim, Ghee Young Kwon, Chan Kyo Kim, Won Park, Hongryull Pyo, Jae Hoon Chung, Wan Song, Hyun Hwan Sung, Byong Chang Jeong, Se Hoon Park
Cancer Res Treat. 2024;56(3):893-897. doi: 10.4143/crt.2024.015.
Reference
-
References
1. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18:329–54.2. Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021; 79:82–104.3. Raj GV, Karavadia S, Schlomer B, Arriaga Y, Lotan Y, Sagalowsky A, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011; 117:276–82.
Article4. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005; 48:202–5.5. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014; 65:350–7.
Article6. Sonpavde G, Goldman BH, Speights VO, Lerner SP, Wood DP, Vogelzang NJ, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009; 115:4104–9.
Article7. Sonpavde G, Khan MM, Lerner SP, Svatek RS, Novara G, Karakiewicz PI, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol. 2011; 185:456–61.
Article8. Sonpavde G. PD-1 and PD-L1 inhibitors as salvage therapy for urothelial carcinoma. N Engl J Med. 2017; 376:1073–4.
Article9. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Luciano R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018; 36:3353–60.
Article10. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019; 25:1706–14.
Article11. Galsky MD, Arija JA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020; 395:1547–57.
Article12. Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021; 22:931–45.13. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017; 18:312–22.
Article14. Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol. 2016; 27:2242–50.
Article15. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011; 29:2432–8.
Article16. Sung HH, Kim H, Kim R, Kim CK, Kwon GY, Park W, et al. Neoadjuvant chemotherapy with gemcitabine and cisplatin followed by selective bladder preservation chemoradiotherapy in muscle-invasive urothelial carcinoma of bladder. Investig Clin Urol. 2022; 63:168–74.
Article17. Cho JH, Kwon GY, Kang M, Sung HH, Jeon HG, Jeong BC, et al. Node-positive bladder cancer after neoadjuvant chemotherapy followed by radical cystectomy: a single-center retrospective study. Korean J Urol Oncol. 2020; 18:194–200.
Article18. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020; 26:1839–44.
Article19. Rose TL, Harrison MR, Deal AM, Ramalingam S, Whang YE, Brower B, et al. Phase II study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer. J Clin Oncol. 2021; 39:3140–8.
Article20. Galsky MD, Daneshmand S, Chan KG, Dorff TB, Cetnar JP, Neil BO, et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer (MIBC): HCRN GU 16–257. J Clin Oncol. 2021; 39(15 Suppl):4503.
Article21. Gupta S, Sonpavde G, Weight CJ, McGregor BA, Gupta S, Maughan BL, et al. Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J Clin Oncol. 2020; 38(6 Suppl):439.
Article22. Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021; 384:2102–14.
Article23. Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016; 6:1382–99.
Article24. Friedman J, Moore EC, Zolkind P, Robbins Y, Clavijo PE, Sun L, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res. 2020; 26:679–89.
Article25. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018; 4:1535–42.
Article26. Chang E, Apolo AB, Bangs R, Chisolm S, Duddalwar V, Efstathiou JA, et al. Refining neoadjuvant therapy clinical trial design for muscle-invasive bladder cancer before cystectomy: a joint US Food and Drug Administration and Bladder Cancer Advocacy Network workshop. Nat Rev Urol. 2022; 19:37–46.
Article27. Niegisch G, Albers P. Which patients benefit the most from neoadjuvant chemotherapy in advanced bladder cancer? Curr Opin Urol. 2011; 21:434–9.
Article28. Martins I, Ribeiro IP, Jorge J, Goncalves AC, Sarmento-Ribeiro AB, Melo JB, et al. Liquid biopsies: applications for cancer diagnosis and monitoring. Genes (Basel). 2021; 12:349.
Article29. Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021; 22:525–37.
Article30. Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021; 595:432–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant chemotherapy with gemcitabine and cisplatin followed by selective bladder preservation chemoradiotherapy in muscle-invasive urothelial carcinoma of bladder
- Chemotherapy in Advanced Urothelial Carcinoma
- Neoadjuvant Cisplatin-Based Chemotherapy Followed by Selective Bladder Preservation Chemoradiotherapy in Muscle-Invasive Urothelial Carcinoma of the Bladder: Post Hoc Analysis of Two Prospective Studies
- Cisplatin, Gemcitabine, and Lapatinib as Neoadjuvant Therapy for Muscle-Invasive Bladder Cancer
- Preliminary Results of Neoadjuvant M-VAC(Methotrexate, Vinblastine, Adriamycin, Cisplatin) Chemotherapy for Bladder Carcinoma