Cancer Res Treat.
2024 Jul;56(3):893-897. 10.4143/crt.2024.015.
Neoadjuvant Cisplatin-Based Chemotherapy Followed by Selective Bladder Preservation Chemoradiotherapy in Muscle-Invasive Urothelial Carcinoma of the Bladder: Post Hoc Analysis of Two Prospective Studies
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Radiology and Center for Imaging Sciences, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2557676
- DOI: http://doi.org/10.4143/crt.2024.015
Abstract
- Purpose
Bladder preservation chemoradiotherapy (CRT) in patients with a clinical complete response (cCR) following cisplatin-based neoadjuvant chemotherapy (NAC) is a promising treatment strategy for muscle-invasive bladder urothelial carcinoma (MIBC). A combined analysis of raw data from two prospective phase II studies was performed to better evaluate the feasibility of selective bladder preservation CRT.
Materials and Methods
The analysis was based on primary efficacy data from two independent studies, including 76 MIBC patients receiving NAC followed by bladder preservation CRT. The efficacy data included metastasis-free survival (MFS) and disease-free survival (DFS). For the present analysis, starting point of survival was defined as the date of commencing CRT.
Results
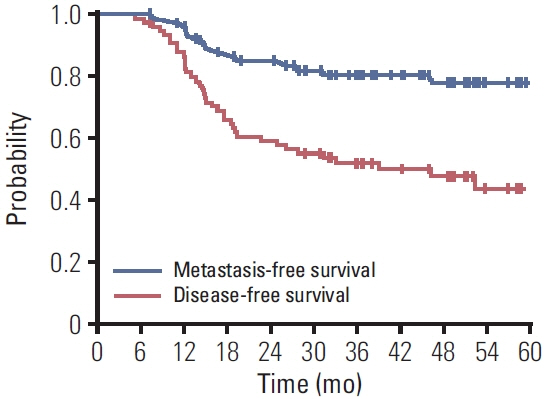
Among 76 patients, 66 had a cCR following NAC. Sixty-four patients received gemcitabine and cisplatin (GC) combination chemotherapy in neoadjuvant setting, and 12 received nivolumab plus GC. Bladder preservation CRT following NAC was generally well-tolerated, with low urinary tract symptoms being the most common late complication. With a median follow-up of 64 months, recurrence was recorded in 43 patients (57%): intravesical only (n=20), metastatic only (n=16), and both (n=7). In 27 patients with intravesical recurrence, transurethral resection, and Bacillus Calmette-Guerin treatment was given to 17 patients. Salvage cystectomy was performed in 10 patients. Median DFS was 46.3 (95% confidence interval [CI], 25.1 to 67.5) months, and the median MFS was not reached. Neither DFS nor MFS appeared to be affected by any of the baseline characteristics. However, DFS was significantly longer in patients with a cCR than in those without (hazard ratio, 0.465; 95% CI, 0.222 to 0.976).
Conclusion
The strategy of NAC followed by selective bladder preservation CRT based on the cCR is feasible in the treatment of MIBC. A standardized definition of cCR is needed to better assess disease status post-NAC.
Figure
Reference
-
References
1. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.
Article2. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18:329–54.3. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003; 349:859–66.
Article4. Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015; 121:2586–93.
Article5. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018; 4:1535–42.
Article6. Ghosh A, Somani BK. Recent trends in postcystectomy healthrelated quality of life (QoL) favors neobladder diversion: systematic review of the literature. Urology. 2016; 93:22–6.
Article7. James N, Hussain SA. Management of muscle invasive bladder cancer: British approaches to organ conservation. Semin Radiat Oncol. 2005; 15:19–27.
Article8. Kozak KR, Hamidi M, Manning M, Moody JS. Bladder preservation for localized muscle-invasive bladder cancer: the survival impact of local utilization rates of definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 83:e197–204.
Article9. Sung HH, Kim H, Kim R, Kim CK, Kwon GY, Park W, et al. Neoadjuvant chemotherapy with gemcitabine and cisplatin followed by selective bladder preservation chemoradiotherapy in muscle-invasive urothelial carcinoma of bladder. Investig Clin Urol. 2022; 63:168–74.
Article10. Kim H, Jeong BC, Hong J, Kwon GY, Kim CK, Park W, et al. Neoadjuvant nivolumab plus gemcitabine/cisplatin chemotherapy in muscle-invasive urothelial carcinoma of the bladder. Cancer Res Treat. 2023; 55:636–42.
Article11. Madersbacher S, Schmidt J, Eberle JM, Thoeny HC, Burkhard F, Hochreiter W, et al. Long-term outcome of ileal conduit diversion. J Urol. 2003; 169:985–90.
Article12. Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014; 32:3801–9.
Article13. Lee HW, Kwon WA, Nguyen NT, Phan DT, Seo HK. Approaches to clinical complete response after neoadjuvant chemotherapy in muscle-invasive bladder cancer: possibilities and limitations. Cancers (Basel). 2023; 15:1323.
Article14. Reese AC, Ball MW, Gandhi N, Gorin MA, Netto GJ, Bivalacqua TJ, et al. The utility of an extensive postchemotherapy staging evaluation in patients receiving neoadjuvant chemotherapy for bladder cancer. Urology. 2014; 84:358–63.
Article15. Becker RE, Meyer AR, Brant A, Reese AC, Biles MJ, Harris KT, et al. Clinical restaging and tumor sequencing are inaccurate indicators of response to neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2021; 79:364–71.
Article16. Geynisman DM, Abbosh P, Ross EA, Zibelman MR, Ghatalia P, Anari F, et al. A phase II trial of risk-enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN). J Clin Oncol. 2023; 41(6 Suppl):438.
Article17. Galsky MD, Daneshmand S, Izadmehr S, Gonzalez-Kozlova E, Chan KG, Lewis S, et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat Med. 2023; 29:2825–34.
Article18. Song SH, Oh JJ. The evolving role of checkpoint inhibitors in the treatment of urothelial carcinoma: a literature review of practice-changing trials. J Urol Oncol. 2023; 21:154–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant chemotherapy with gemcitabine and cisplatin followed by selective bladder preservation chemoradiotherapy in muscle-invasive urothelial carcinoma of bladder
- Combined Modality Therapy with Selective Bladder Preservation for Muscle Invading Bladder Cancer
- Bladder Preservation Management for Muscle Invasive Bladder Cancer
- Preliminary Results of Neoadjuvant M-VAC(Methotrexate, Vinblastine, Adriamycin, Cisplatin) Chemotherapy for Bladder Carcinoma
- Bladder Preservation Strategies for Treatment of Muscle-Invasive Bladder Cancer: Experience of 7-Year Follow up