J Korean Med Sci.
2023 Feb;38(8):e59. 10.3346/jkms.2023.38.e59.
Short-Term Effectiveness of Oral Nirmatrelvir/Ritonavir Against the SARS-CoV-2 Omicron Variant and Culture-Positive Viral Shedding
- Affiliations
-
- 1Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 2National Institute of Infectious Diseases, Korea National Institute of Health, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 3Division of Infectious Diseases, Seoul Medical Center, Seoul, Korea
- 4Division of Infectious Diseases, National Medical Center, Seoul, Korea
- 5Seoul Veterans Hospital Medical Center, Seoul, Korea
- 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, National Medical Center, Seoul, Korea
- KMID: 2539632
- DOI: http://doi.org/10.3346/jkms.2023.38.e59
Abstract
- Background
Information on the effectiveness of nirmatrelvir/ritonavir against the omicron is limited. The clinical response and viral kinetics to therapy in the real world need to be evaluated.
Methods
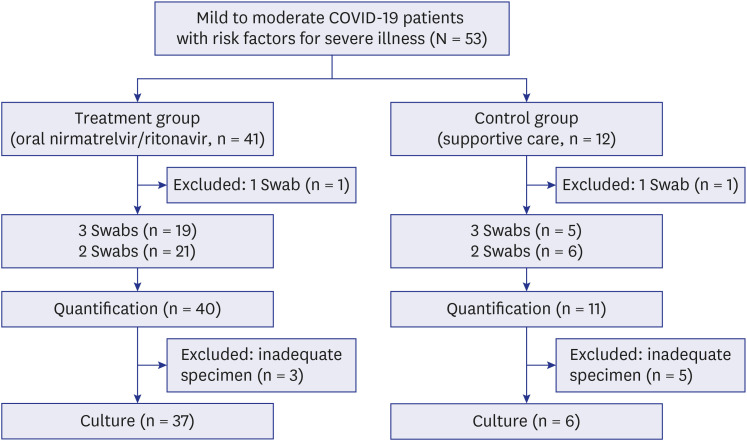
Mild to moderate coronavirus disease 2019 (COVID-19) patients with risk factors for severe illness were prospectively enrolled as a treatment group with nirmatrelvir/ritonavir therapy versus a control group with supportive care. Serial viral load and culture from the upper respiratory tract were evaluated for seven days, and clinical responses and adverse reactions were evaluated for 28 days.
Results
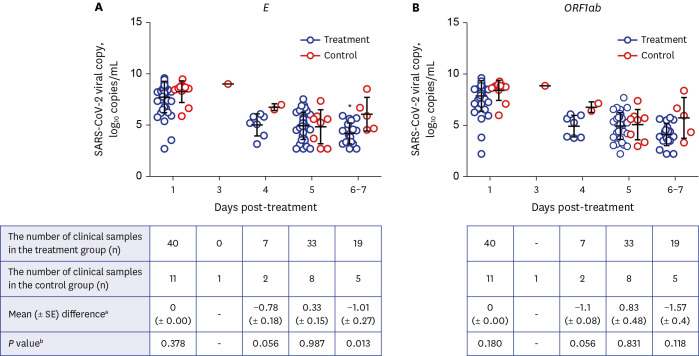
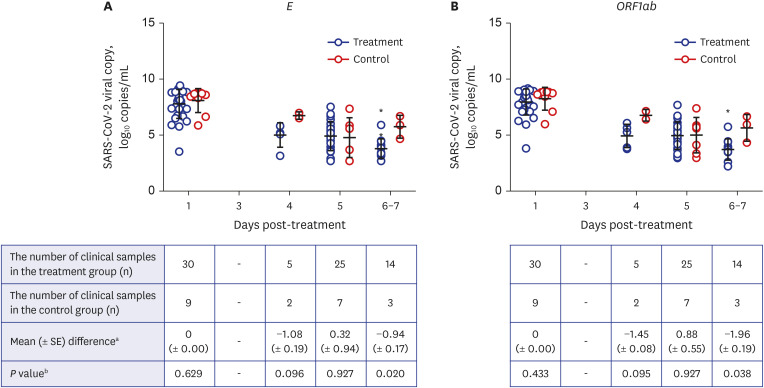
A total of 51 patients were analyzed including 40 in the treatment group and 11 in the control group. Faster symptom resolution during hospitalization (P= 0.048) was observed in the treatment group. Only minor adverse reactions were reported in 27.5% of patients. The viral load on Day 7 was lower in the treatment group (P = 0.002). The viral culture showed a positivity of 67.6% (25/37) vs. 100% (6/6) on Day 1, 0% (0/37) vs. 16.7 (1/6) on Day 5, and 0% (0/16) vs. 50.0% (2/4) on Day 7 in the treatment and control groups, respectively.
Conclusions
Nirmatrelvir/ritonavir against the omicron was safe and resulted in negative viral culture conversion after Day 5 of treatment with better symptomatic resolution.
Keyword
Figure
Reference
-
2. Ranney ML, Griffeth V, Jha AK. Critical supply shortages-the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020; 382(18):e41. PMID: 32212516.3. Feral-Pierssens AL, Claret PG, Chouihed T. Collateral damage of the COVID-19 outbreak: expression of concern. Eur J Emerg Med. 2020; 27(4):233–234. PMID: 32345850.
Article4. Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021; 374(6575):1586–1593. PMID: 34726479.
Article5. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, non-hospitalized adults with COVID-19. N Engl J Med. 2022; 386(15):1397–1408. PMID: 35172054.
Article6. Ganatra S, Dani SS, Ahmad J, Kumar A, Shah J, Abraham GM, et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin Infect Dis. 2022; ciac673.7. Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2023; 76(3):e342–e349. PMID: 35653428.8. Wong CK, Au IC, Lau KT, Lau EH, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022; 22(12):1681–1693. PMID: 36029795.
Article9. Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022; 387(9):790–798. PMID: 36001529.
Article10. Korea Disease Control and Prevention Agency. Instructions for use of COVID-19 treatment (version 4-3). Updated 2022. Accessed October 12, 2022. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=718756&nPage=1 .11. Central Disaster Management Headquarters. Korean Guideline Recommendation for treatment of COVID-19 (7-2). Updated 2022. Accessed October 12, 2022. https://www.kdca.go.kr/board/boardApi.es?mid=a20507050000&bid=0080 .12. Wong D, Bonnici T, Knight J, Gerry S, Turton J, Watkinson P. A ward-based time study of paper and electronic documentation for recording vital sign observations. J Am Med Inform Assoc. 2017; 24(4):717–721. PMID: 28339626.
Article13. Savarese DM. Common Terminology Criteria for Adverse Events (CTCAE). Waltham, MA, USA: UpToDate;2013. p. 1–9.14. Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020; 383(26):2586–2588. PMID: 33259154.
Article15. Kim MC, Cui C, Shin KR, Bae JY, Kweon OJ, Lee MK, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19. N Engl J Med. 2021; 384(7):671–673. PMID: 33503337.16. Keske Ş, Güney-Esken G, Vatansever C, Beşli Y, Kuloğlu ZE, Nergiz Z, et al. Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms. Clin Microbiol Infect. 2022; S1198-743X(22)00373-1.
Article17. Jung J, Kim JY, Park H, Park S, Lim JS, Lim SY, et al. Transmission and infectious SARS-CoV-2 shedding kinetics in vaccinated and unvaccinated Individuals. JAMA Netw Open. 2022; 5(5):e2213606. PMID: 35608859.18. Takahashi K, Ishikane M, Ujiie M, Iwamoto N, Okumura N, Sato T, et al. Duration of infectious virus shedding by SARS-CoV-2 Omicron variant–infected vaccinees. Emerg Infect Dis. 2022; 28(5):998–1001. PMID: 35290176.19. Lee CM, Lee E, Park WB, Choe PG, Song KH, Kim ES, et al. Breakthrough COVID-19 infection during the delta variant dominant period: individualized care based on vaccination status is needed. J Korean Med Sci. 2022; 37(32):e252. PMID: 35971766.20. Centers for Disease Control and Prevention. CDC updates and shortens recommended isolation and quarantine period for general population. Update 2021. Accessed October 12, 2022. https://www.cdc.gov/media/releases/2021/s1227-isolation-quarantine-guidance.html .21. Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020; 382(22):2081–2090. PMID: 32329971.
Article22. Central Disaster Management Headquarters. Guidelines of COVID-19 management for local government. 13th edition. Updated 2022. Accessed October 12, 2022. https://ncov.kdca.go.kr/duBoardList.do?brdId=2&brdGubun=28 .23. Boucau J, Marino C, Regan J, Uddin R, Choudhary MC, Flynn JP, et al. Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection. N Engl J Med. 2022; 387(3):275–277. PMID: 35767428.
Article24. Li P, Wang Y, Lavrijsen M, Lamers MM, de Vries AC, Rottier RJ, et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022; 32(3):322–324. PMID: 35058606.
Article25. Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022; 386(15):1475–1477. PMID: 35263535.
Article26. Ranganath N, O’Horo JC, Challener DW, Tulledge-Scheitel SM, Pike ML, Michael O’Brien R, et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2023; 76(3):e537–e539. PMID: 35698452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Paxlovid in COVID-19 treatment during the periods of SARS-CoV-2 Omicron BA.5 and BN.1 subvariant dominance in the Republic of Korea: a retrospective cohort study
- Analysis of SARS-CoV-2 Mutations after Nirmatrelvir Treatment in a Lung Cancer Xenograft Mouse Model
- Reinfection of SARS-CoV-2 Variants in Immunocompromised Patients with Prolonged or Relapsed Viral Shedding
- Recombinant proteins of spike protein of SARS-CoV-2 with the Omicron receptor-binding domain induce production of highly Omicron-specific neutralizing antibodies
- Viral shedding patterns of symptomatic SARS-CoV-2 infections by periods of variant predominance and vaccination status in Gyeonggi Province, Korea