Int J Stem Cells.
2023 Feb;16(1):44-51. 10.15283/ijsc22138.
Embryonic Stem Cells Lacking DNA Methyltransferases Differentiate into Neural Stem Cells that Are Defective in Self-Renewal
- Affiliations
-
- 1Department of Stem Cell and Regenerative Biotechnology, Konkuk Institute of Technology, Konkuk University, Seoul, Korea
- 23D Tissue Culture Research Center, Konkuk University, Seoul, Korea
- 3Department of Animal Science, Sangji University, Wonju, Korea
- 4Department of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, Münster, Germany
- KMID: 2539620
- DOI: http://doi.org/10.15283/ijsc22138
Abstract
- Background and Objectives
DNA methyltransferases (Dnmts) play an important role in regulating DNA methylation during early developmental processes and cellular differentiation. In this study, we aimed to investigate the role of Dnmts in neural differentiation of embryonic stem cells (ESCs) and in maintenance of the resulting neural stem cells (NSCs).
Methods and Results
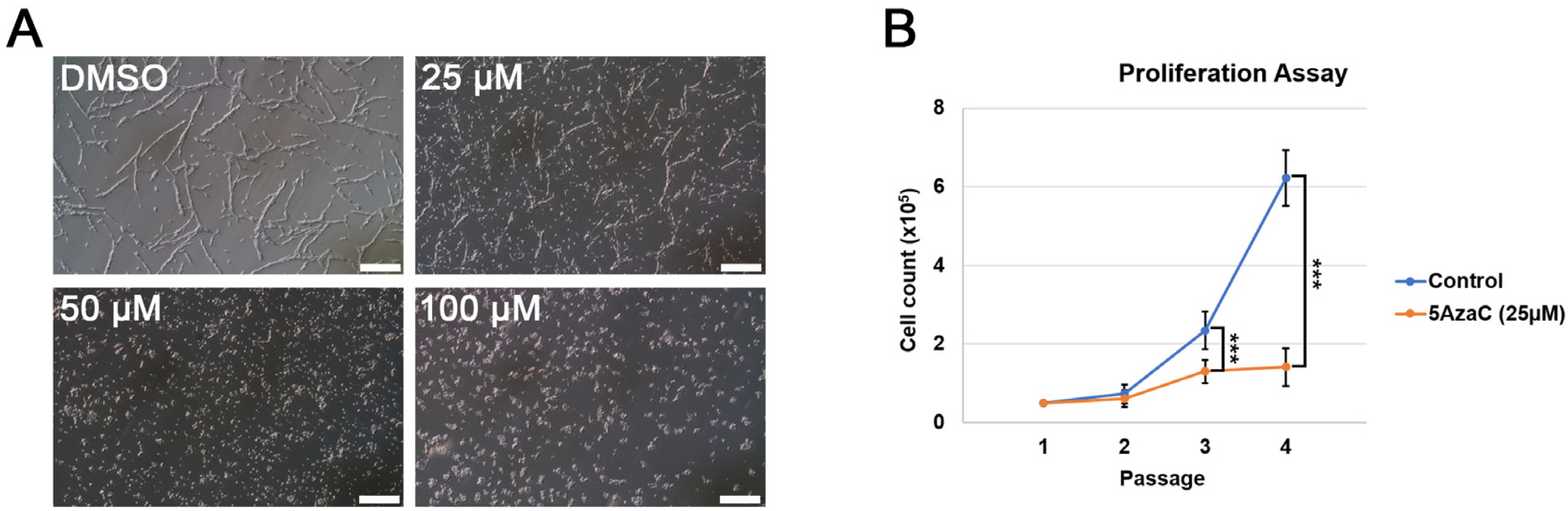
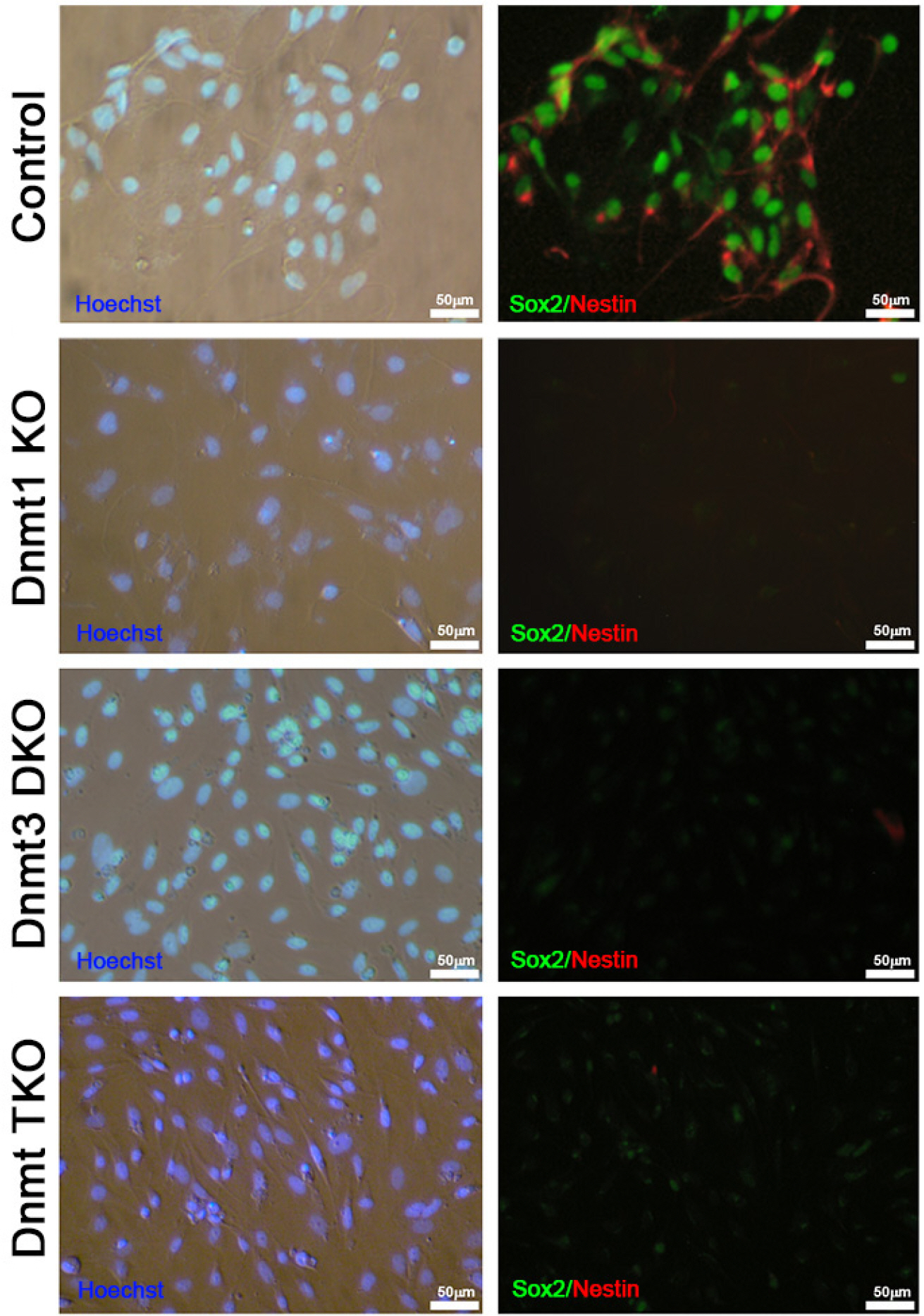
We used three types of Dnmt knockout (KO) ESCs, including Dnmt1 KO, Dnmt3a/3b double KO (Dnmt3 DKO), and Dnmt1/3a/3b triple KO (Dnmt TKO), to investigate the role of Dnmts in neural differentiation of ESCs. All three types of Dnmt KO ESCs could form neural rosette and differentiate into NSCs in vitro. Interestingly, however, after passage three, Dnmt KO ESC-derived NSCs could not maintain their self-renewal and differentiated into neurons and glial cells.
Conclusions
Taken together, the data suggested that, although deficiency of Dnmts had no effect on the differentiation of ESCs into NSCs, the latter had defective maintenance, thereby indicating that Dnmts are crucial for self-renewal of NSCs.
Figure
Reference
-
References
1. Zemach A, McDaniel IE, Silva P, Zilberman D. 2010; Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 328:916–919. DOI: 10.1126/science.1186366. PMID: 20395474.2. Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D. 2007; Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 39:457–466. DOI: 10.1038/ng1990. PMID: 17334365.3. Leonhardt H, Page AW, Weier HU, Bestor TH. 1992; A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 71:865–873. DOI: 10.1016/0092-8674(92)90561-P. PMID: 1423634.4. Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. 2008; DNA methylation inhibitor 5-Aza-2'-deoxy-cytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 28:752–771. DOI: 10.1128/MCB.01799-07. PMID: 17991895. PMCID: PMC2223421.5. Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li E, Ueda HR, Nakayama J, Okano M. 2006; Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 11:805–814. DOI: 10.1111/j.1365-2443.2006.00984.x. PMID: 16824199.6. Bronner C. 2011; Control of DNMT1 abundance in epigenetic inheritance by acetylation, ubiquitylation, and the histone code. Sci Signal. 4:pe3. DOI: 10.1126/scisignal.2001764. PMID: 21266713.7. Okano M, Bell DW, Haber DA, Li E. 1999; DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 99:247–257. DOI: 10.1016/S0092-8674(00)81656-6. PMID: 10555141.8. Hu YG, Hirasawa R, Hu JL, Hata K, Li CL, Jin Y, Chen T, Li E, Rigolet M, Viegas-Péquignot E, Sasaki H, Xu GL. 2008; Regulation of DNA methylation activity through Dnmt3L promoter methylation by Dnmt3 enzymes in embryonic development. Hum Mol Genet. 17:2654–2664. DOI: 10.1093/hmg/ddn165. PMID: 18544626.9. Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. 2007; Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet. 16:2272–2280. DOI: 10.1093/hmg/ddm179. PMID: 17616512.10. Chédin F. 2011; The DNMT3 family of mammalian de novo DNA methyltransferases. Prog Mol Biol Transl Sci. 101:255–285. DOI: 10.1016/B978-0-12-387685-0.00007-X. PMID: 21507354.11. Xiao Y, Word B, Starlard-Davenport A, Haefele A, Lyn-Cook BD, Hammons G. 2008; Age and gender affect DNMT3a and DNMT3b expression in human liver. Cell Biol Toxicol. 24:265–272. DOI: 10.1007/s10565-007-9035-9. PMID: 17929180.12. Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. 2010; DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 463:563–567. DOI: 10.1038/nature08683. PMID: 20081831. PMCID: PMC3050546.13. Georgia S, Kanji M, Bhushan A. 2013; DNMT1 represses p53 to maintain progenitor cell survival during pancreatic organo-genesis. Genes Dev. 27:372–377. DOI: 10.1101/gad.207001.112. PMID: 23431054. PMCID: PMC3589554.14. Ramesh V, Bayam E, Cernilogar FM, Bonapace IM, Schulze M, Riemenschneider MJ, Schotta G, Götz M. 2016; Loss of Uhrf1 in neural stem cells leads to activation of retrovi-ral elements and delayed neurodegeneration. Genes Dev. 30:2199–2212. DOI: 10.1101/gad.284992.116. PMID: 27798843. PMCID: PMC5088568.15. Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, Liang S, Lu Y, Darlington GJ, Meissner A, Issa JP, Godley LA, Li W, Goodell MA. 2011; Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 44:23–31. DOI: 10.1038/ng.1009. PMID: 22138693. PMCID: PMC3637952.16. Bröske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R, Nerlov C, Leutz A, Andrade-Navarro MA, Jacobsen SE, Rosenbauer F. 2009; DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 41:1207–1215. DOI: 10.1038/ng.463. PMID: 19801979.17. Sato A, Sunayama J, Matsuda K, Tachibana K, Sakurada K, Tomiyama A, Kayama T, Kitanaka C. 2010; Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett. 470:115–120. DOI: 10.1016/j.neulet.2009.12.067. PMID: 20045038.18. Campos LS, Leone DP, Relvas JB, Brakebusch C, Fässler R, Suter U, ffrench-Constant C. 2004; Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 131:3433–3444. DOI: 10.1242/dev.01199. PMID: 15226259.19. Braunschweig L, Meyer AK, Wagenführ L, Storch A. 2015; Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol Cell Neurosci. 67:84–92. DOI: 10.1016/j.mcn.2015.06.006. PMID: 26079803.20. Ehm O, Göritz C, Covic M, Schäffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisén J, Lie DC. 2010; RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 30:13794–13807. DOI: 10.1523/JNEUROSCI.1567-10.2010. PMID: 20943920. PMCID: PMC6633732.21. Ladd-Acosta C, Pevsner J, Sabunciyan S, Yolken RH, Webster MJ, Dinkins T, Callinan PA, Fan JB, Potash JB, Feinberg AP. 2007; DNA methylation signatures within the human brain. Am J Hum Genet. 81:1304–1315. DOI: 10.1086/524110. PMID: 17999367. PMCID: PMC2276356.22. Jang HS, Shin WJ, Lee JE, Do JT. 2017; CpG and Non-CpG methylation in epigenetic gene regulation and brain function. Genes (Basel). 8:148. DOI: 10.3390/genes8060148. PMID: 28545252. PMCID: PMC5485512.23. Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, Pop R, Reyon D, Tsai SQ, Mallard W, Joung JK, Rinn JL, Gnirke A, Meissner A. 2015; Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet. 47:469–478. DOI: 10.1038/ng.3258. PMID: 25822089. PMCID: PMC4414868.24. Biniszkiewicz D, Gribnau J, Ramsahoye B, Gaudet F, Eggan K, Humpherys D, Mastrangelo MA, Jun Z, Walter J, Jaenisch R. 2002; Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 22:2124–2135. DOI: 10.1128/MCB.22.7.2124-2135.2002. PMID: 11884600. PMCID: PMC133685.25. Choi HW, Kim JS, Choi S, Hong YJ, Kim MJ, Seo HG, Do JT. 2014; Neural stem cells differentiated from iPS cells spontaneously regain pluripotency. Stem Cells. 32:2596–2604. DOI: 10.1002/stem.1757. PMID: 24898298.26. Müller AM, Florek M. 2014; 5-Azacytidine/5-Azacitidine. Recent Results Cancer Res. 201:299–324. DOI: 10.1007/978-3-642-54490-3_19. PMID: 24756801.27. Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. 2005; Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 3:e283. DOI: 10.1371/journal.pbio.0030283. PMID: 16086633. PMCID: PMC1184591. PMID: 12caa13fe1cc4e2781031e1d2981ad9c.28. Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC, Jaenisch R. 2011; Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 9:166–175. DOI: 10.1016/j.stem.2011.07.010. PMID: 21816367. PMCID: PMC3154739.29. Li T, Yang D, Li J, Tang Y, Yang J, Le W. 2015; Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol Neurobiol. 51:142–154. DOI: 10.1007/s12035-014-8734-5. PMID: 24838624.30. Seo BJ, Choi J, La H, Habib O, Choi Y, Hong K, Do JT. 2020; Role of mitochondrial fission-related genes in mitochondrial morphology and energy metabolism in mouse embryonic stem cells. Redox Biol. 36:101599. DOI: 10.1016/j.redox.2020.101599. PMID: 32521505. PMCID: PMC7286981.31. Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. 2007; Nanog safeguards pluripotency and mediates germline development. Nature. 450:1230–1234. DOI: 10.1038/nature06403. PMID: 18097409.32. Tateishi S, Niwa H, Miyazaki J, Fujimoto S, Inoue H, Yamaizumi M. 2003; Enhanced genomic instability and defective postreplication repair in RAD18 knockout mouse embryonic stem cells. Mol Cell Biol. 23:474–481. DOI: 10.1128/MCB.23.2.474-481.2003. PMID: 12509447. PMCID: PMC151530.33. Langton S, Gudas LJ. 2008; CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Dev Biol. 315:331–354. DOI: 10.1016/j.ydbio.2007.12.021. PMID: 18241852.34. Murao N, Noguchi H, Nakashima K. 2016; Epigenetic regulation of neural stem cell property from embryo to adult. Neuroe-pigenetics. 5:1–10. DOI: 10.1016/j.nepig.2016.01.001.35. Noguchi H, Kimura A, Murao N, Matsuda T, Namihira M, Nakashima K. 2015; Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neurosci Res. 95:1–11. DOI: 10.1016/j.neures.2015.01.014. PMID: 25659757.36. Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. 2010; Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 329:444–448. DOI: 10.1126/science.1190485. PMID: 20651149. PMCID: PMC3539760.37. Gaiano N, Fishell G. 2002; The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 25:471–490. DOI: 10.1146/annurev.neuro.25.030702.130823. PMID: 12052917.38. Aguirre A, Rubio ME, Gallo V. 2010; Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 467:323–327. DOI: 10.1038/nature09347. PMID: 20844536. PMCID: PMC2941915.39. Engler A, Rolando C, Giachino C, Saotome I, Erni A, Brien C, Zhang R, Zimber-Strobl U, Radtke F, Artavanis-Tsakonas S, Louvi A, Taylor V. 2018; Notch2 signaling maintains NSC quiescence in the murine ventricular-subventricular zone. Cell Rep. 22:992–1002. DOI: 10.1016/j.celrep.2017.12.094. PMID: 29386140.40. Valvezan AJ, Klein PS. 2012; GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Front Mol Neurosci. 5:1. DOI: 10.3389/fnmol.2012.00001. PMID: 22319467. PMCID: PMC3268224.41. Lim DA, Tramontin AD, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. 2000; Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 28:713–726. DOI: 10.1016/S0896-6273(00)00148-3. PMID: 11163261.42. Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL. 2013; Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 13:237–245. DOI: 10.1016/j.stem.2013.05.006. PMID: 23770080. PMCID: PMC4474382.43. Jobe EM, Gao Y, Eisinger BE, Mladucky JK, Giuliani CC, Kelnhofer LE, Zhao X. 2017; Methyl-CpG-binding protein MBD1 regulates neuronal lineage commitment through maintaining adult neural stem cell identity. J Neurosci. 37:523–536. DOI: 10.1523/JNEUROSCI.1075-16.2016. PMID: 28100736. PMCID: PMC5242405.44. Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. 2010; Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 6:433–444. DOI: 10.1016/j.stem.2010.02.017. PMID: 20452318. PMCID: PMC2867837.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts of Stem Cell Therapy
- Cell Biological Characteristics of Adult Stem Cells
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Regulation of Neural Stem Cell Fate by Natural Products
- Novel Technique for Inducing Neural Crest Fate in Embryonic Stem Cells (Stem Cells 2009;27:2896-2905)