Diabetes Metab J.
2023 Jan;47(1):104-117. 10.4093/dmj.2022.0081.
Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University Gwangmyeong Hospital, Chung-Ang University College of Medicine, Gwangmyeong, Korea
- 2Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- 3Department of Health Screening and Promotion Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Asan Diabetes Center, Asan Medical Center, Seoul, Korea
- KMID: 2538935
- DOI: http://doi.org/10.4093/dmj.2022.0081
Abstract
- Background
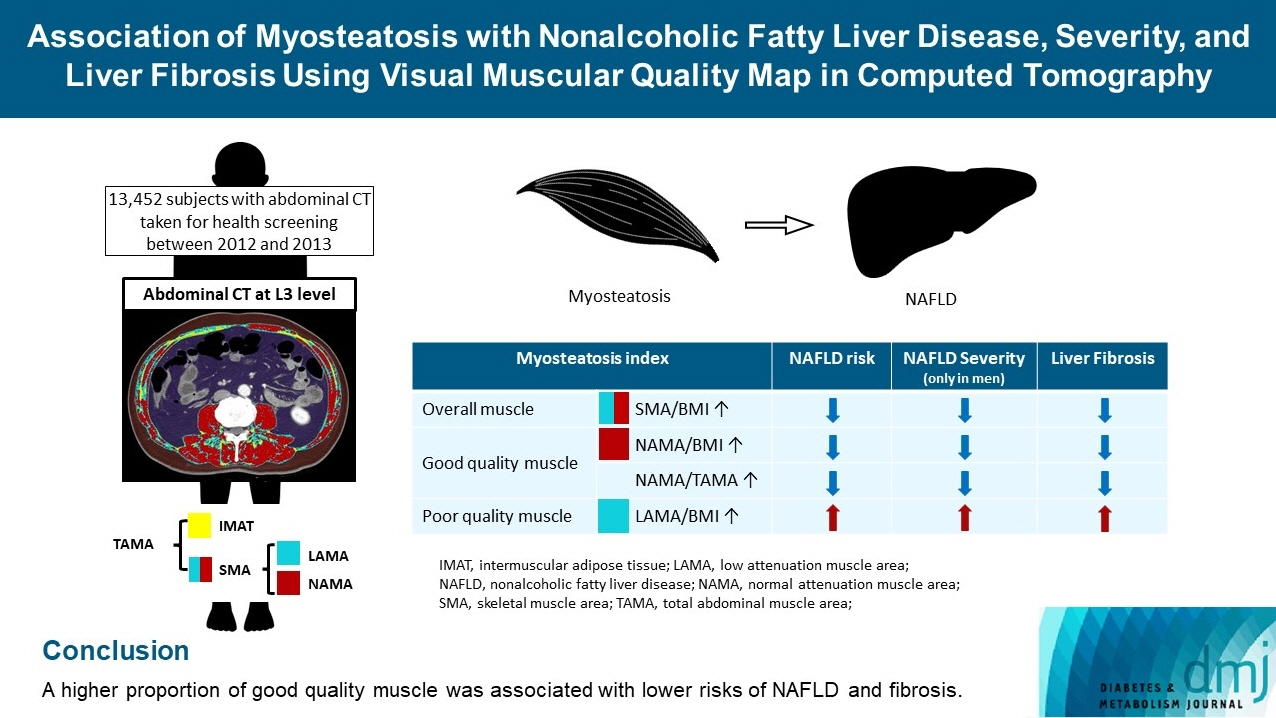
The association of myosteatosis measured using visual muscular quality map in computed tomography (CT) with nonalcoholic fatty liver disease (NAFLD), its severity, and fibrosis was analyzed in a large population.
Methods
Subjects (n=13,452) with abdominal CT between 2012 and 2013 were measured total abdominal muscle area (TAMA) at L3 level. TAMA was segmented into intramuscular adipose tissue and skeletal muscle area (SMA), which was further classified into normal attenuation muscle area (NAMA) and low attenuation muscle area (LAMA). The following variables were adopted as indicators of myosteatosis: SMA/body mass index (BMI), NAMA/BMI, NAMA/TAMA, and LAMA/BMI. NAFLD and its severity were assessed by ultrasonography, and liver fibrosis was measured by calculating the NAFLD fibrosis score (NFS) and fibrosis-4 index (FIB-4) scores.
Results
According to multiple logistic regression analyses, as quartiles of SMA/BMI, NAMA/BMI, and NAMA/TAMA increased, the odds ratios (ORs) for NAFLD decreased in each sex (P for trend <0.001 for all). The ORs of moderate/severe NAFLD were significantly higher in the Q1 group than in the Q4 group for SMA/BMI, NAMA/BMI, and NAMA/TAMA in men. The ORs of intermediate/high liver fibrosis scores assessed by NFS and FIB-4 scores increased linearly with decreasing quartiles for SMA/BMI, NAMA/BMI, and NAMA/TAMA in each sex (P for trend <0.001 for all). Conversely, the risk for NAFLD and fibrosis were positively associated with LAMA/BMI quartiles in each sex (P for trend <0.001 for all).
Conclusion
A higher proportion of good quality muscle was associated with lower risks of NAFLD and fibrosis.
Keyword
Figure
Reference
-
1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article2. Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011). Hepatology. 2016; 63:776–86.
Article3. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017; 66:123–31.
Article4. Park SJ, Ryu SY, Park J, Choi SW. Association of sarcopenia with metabolic syndrome in Korean population using 2009-2010 Korea National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2019; 17:494–9.
Article5. Cho HW, Chung W, Moon S, Ryu OH, Kim MK, Kang JG. Effect of sarcopenia and body shape on cardiovascular disease according to obesity phenotypes. Diabetes Metab J. 2021; 45:209–18.
Article6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31.
Article7. Fragala MS, Kenny AM, Kuchel GA. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med. 2015; 45:641–58.
Article8. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014; 2014:309570.
Article9. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014; 210:489–97.
Article10. Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019; 20:205–17.
Article11. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011; 54:1082–90.
Article12. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007; 45:846–54.
Article13. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009; 7:1104–12.
Article14. Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020; 158:1999–2014.
Article15. Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020; 17:387–8.
Article16. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014; 59:1772–8.
Article17. Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015; 63:486–93.
Article18. Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring). 2009; 17:1062–9.
Article19. Kim SK, Park SW, Hwang IJ, Lee YK, Cho YW. High fat stores in ectopic compartments in men with newly diagnosed type 2 diabetes: an anthropometric determinant of carotid atherosclerosis and insulin resistance. Int J Obes (Lond). 2010; 34:105–10.
Article20. Terry JG, Shay CM, Schreiner PJ, Jacobs DR Jr, Sanchez OA, Reis JP, et al. Intermuscular adipose tissue and subclinical coronary artery calcification in midlife: the CARDIA Study (Coronary Artery Risk Development in Young Adults). Arterioscler Thromb Vasc Biol. 2017; 37:2370–8.21. Lee MJ, Kim HK, Kim EH, Bae SJ, Kim KW, Kim MJ, et al. Association between muscle quality measured by abdominal computed tomography and subclinical coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2021; 41:e128–40.
Article22. Tanaka M, Okada H, Hashimoto Y, Kumagai M, Nishimura H, Oda Y, et al. Relationship between nonalcoholic fatty liver disease and muscle quality as well as quantity evaluated by computed tomography. Liver Int. 2020; 40:120–30.
Article23. Hsieh YC, Joo SK, Koo BK, Lin HC, Kim W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021; 41:494–504.
Article24. Kim EH, Kim KW, Shin Y, Lee J, Ko Y, Kim YJ, et al. Reference data and T-scores of lumbar skeletal muscle area and its skeletal muscle indices measured by CT scan in a healthy Korean population. J Gerontol A Biol Sci Med Sci. 2021; 76:265–71.
Article25. Kim HK, Kim KW, Kim EH, Lee MJ, Bae SJ, Ko Y, et al. Age-related changes in muscle quality and development of diagnostic cutoff points for myosteatosis in lumbar skeletal muscles measured by CT scan. Clin Nutr. 2021; 40:4022–8.
Article26. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014; 69:567–75.
Article27. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014; 371:1131–41.
Article28. Chakravarthy MV, Siddiqui MS, Forsgren MF, Sanyal AJ. Harnessing muscle-liver crosstalk to treat nonalcoholic steatohepatitis. Front Endocrinol (Lausanne). 2020; 11:592373.
Article29. Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring). 2008; 16:1394–9.
Article30. Kob R, Bollheimer LC, Bertsch T, Fellner C, Djukic M, Sieber CC, et al. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology. 2015; 16:15–29.
Article31. Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005; 82:428–34.32. Kim JA, Choi KM. Sarcopenia and fatty liver disease. Hepatol Int. 2019; 13:674–87.
Article33. Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7-year longitudinal study. Hepatology. 2018; 68:1755–68.
Article34. Petta S, Ciminnisi S, Di Marco V, Cabibi D, Camma C, Licata A, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017; 45:510–8.
Article35. Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019; 31:1121–8.
Article36. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017; 34:1291–326.
Article37. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019; 70:1457–69.
Article38. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015; 386:266–73.
Article39. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020; 8:616–27.
Article40. Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012; 10:166–73.
Article41. Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013; 47:861–70.
Article42. Chan WK, Treeprasertsuk S, Goh GB, Fan JG, Song MJ, Charatcharoenwitthaya P, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2019; 17:2570–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography (Diabetes Metab J 2023;47:104-17)
- Association of Myosteatosis with Nonalcoholic Fatty Liver Disease, Severity, and Liver Fibrosis Using Visual Muscular Quality Map in Computed Tomography (Diabetes Metab J 2023;47:104-17)
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- Hepatic and Splenic Volumetry Could Be Used as an Imaging Parameter to Evaluate Fibrosis Grades of the Diffuse Liver Disease Including Nonalcoholic Fatty Liver Disease
- Noninvasive diagnosis of pediatric nonalcoholic fatty liver disease