Cancer Res Treat.
2023 Jan;55(1):279-290. 10.4143/crt.2022.073.
Epidemiologic and Clinical Outcomes of Pediatric Renal Tumors in Korea: A Retrospective Analysis of The Korean Pediatric Hematology and Oncology Group (KPHOG) Data
- Affiliations
-
- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Pediatric Hematology and Oncology, Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 4Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- 5Seoul National University Cancer Institute, Seoul, Korea
- 6Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Pediatrics, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Gwangju, Korea
- 8Department of Pediatrics, Pusan National University School of Medicine, Yangsan, Korea
- 9Department of Pediatrics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 10Center for Pediatric Cancer, Department of Pediatrics, National Cancer Center, Goyang, Korea
- 11Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea
- 12Departments of Pediatrics, Korea University College of Medicine, Seoul, Korea
- 13Department of Pediatrics, College of Medicine, Inha University Hospital, Incheon, Korea
- 14Department of Pediatrics, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea
- 15Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea
- 16Department of Pediatrics and Research Institute of Clinical Medicine of Jeonbuk National University-Jeonbuk National University Hospital, Jeonbuk National University Medical School, Jeonju, Korea
- 17Department of Pediatrics, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 18Department of Pediatrics, Gyeongsang National University School of Medicine, Jinju, Korea
- 19Department of Pediatrics, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 20Department of Pediatrics, Ewha Womans University Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Korea
- 21Department of Pediatrics, Chungnam National University College of Medicine, Daejeon, Korea
- 22Department of Pediatrics, Kyung Hee University College of Medicine, Seoul, Korea
- 23Department of Pediatrics, Dankook University College of Medicine, Cheonan, Korea
- 24Department of Pediatrics, Yeungnam University College of Medicine, Daegu, Korea
- 25Department of Pediatrics, Gachon University Gil Medical Center, Incheon, Korea
- 26Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 27Department of Pediatrics, Dong-A University College of Medicine, Busan, Korea
- 28Medical Research Collaborating Center, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2538011
- DOI: http://doi.org/10.4143/crt.2022.073
Abstract
- Purpose
Renal tumors account for approximately 7% of all childhood cancers. These include Wilms tumor (WT), clear cell sarcoma of the kidney (CCSK), malignant rhabdoid tumor of the kidney (MRTK), renal cell carcinoma (RCC), congenital mesoblastic nephroma (CMN) and other rare tumors. We investigated the epidemiology of pediatric renal tumors in Korea.
Materials and Methods
From January 2001 to December 2015, data of pediatric patients (0–18 years) newly-diagnosed with renal tumors at 26 hospitals were retrospectively analyzed.
Results
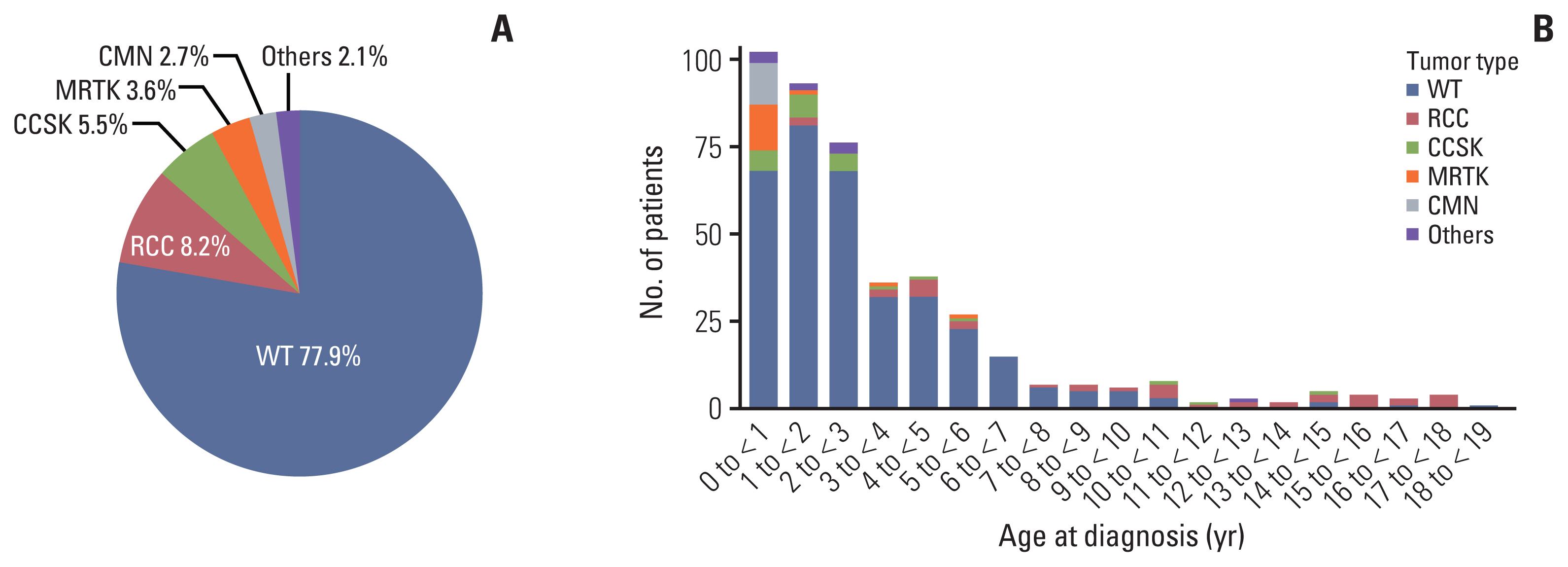
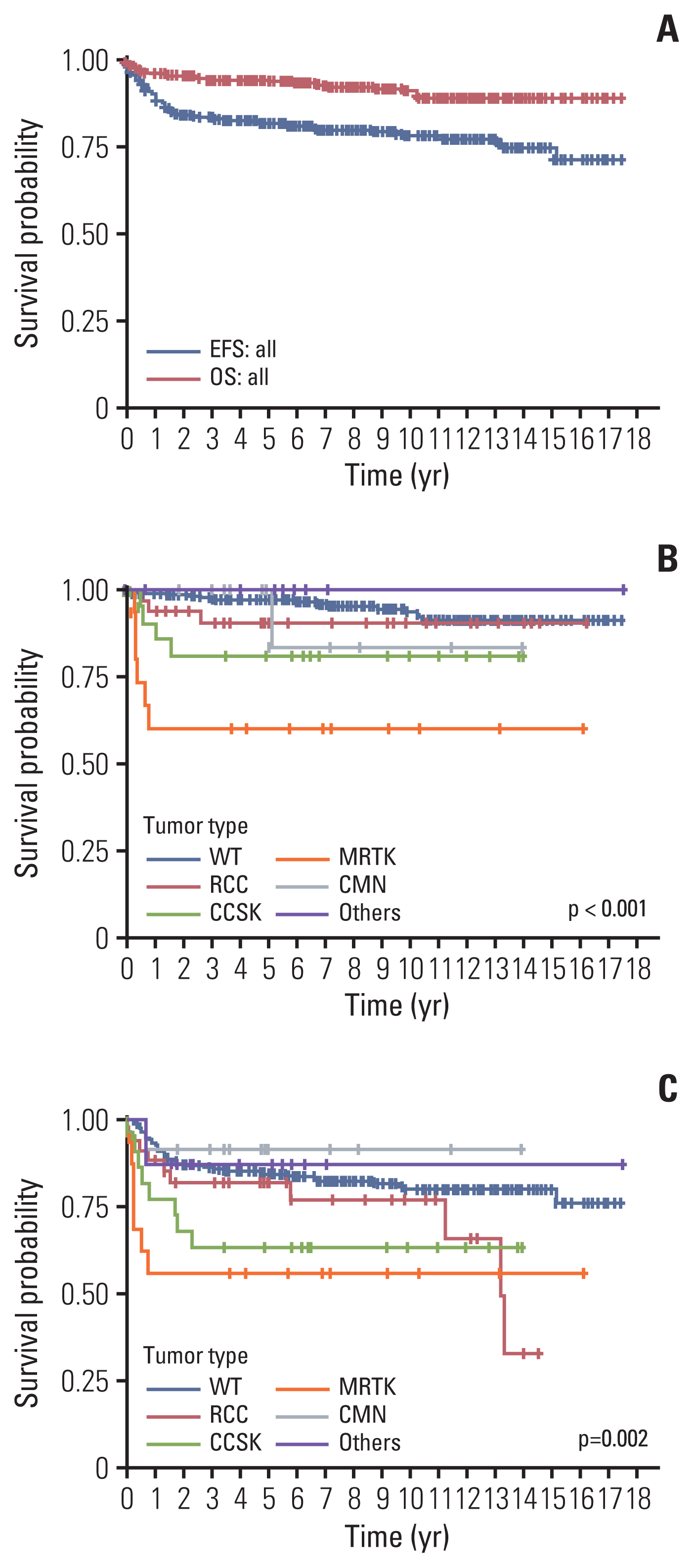
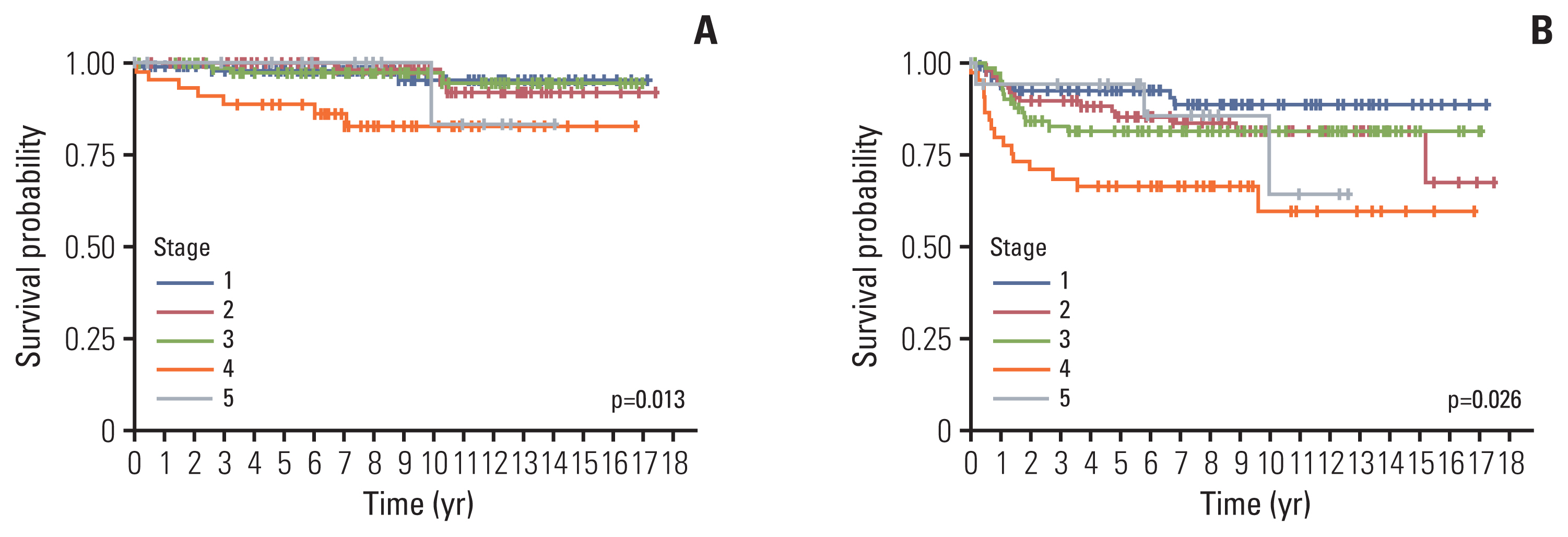
Among 439 patients (male, 240), the most common tumor was WT (n=342, 77.9%), followed by RCC (n=36, 8.2%), CCSK (n=24, 5.5%), MRTK (n=16, 3.6%), CMN (n=12, 2.7%), and others (n=9, 2.1%). Median age at diagnosis was 27.1 months (range 0-225.5) and median follow-up duration was 88.5 months (range 0-211.6). Overall, 32 patients died, of whom 17, 11, 1, and 3 died of relapse, progressive disease, second malignant neoplasm, and treatment-related mortality. Five-year overall survival and event free survival were 97.2% and 84.8% in WT, 90.6% and 82.1% in RCC, 81.1% and 63.6% in CCSK, 60.3% and 56.2% in MRTK, and 100% and 91.7% in CMN, respectively (p < 0.001).
Conclusion
The pediatric renal tumor types in Korea are similar to those previously reported in other countries. WT accounted for a large proportion and survival was excellent. Non-Wilms renal tumors included a variety of tumors and showed inferior outcome, especially MRTK. Further efforts are necessary to optimize the treatment and analyze the genetic characteristics of pediatric renal tumors in Korea.
Keyword
Figure
Reference
-
References
1. Brok J, Treger TD, Gooskens SL, van den Heuvel-Eibrink MM, Pritchard-Jones K. Biology and treatment of renal tumours in childhood. Eur J Cancer. 2016; 68:179–95.2. Chung EM, Graeber AR, Conran RM. Renal tumors of childhood: radiologic-pathologic correlation part 1. The 1st decade: from the radiologic pathology archives. Radiographics. 2016; 36:499–522.3. Nakata K, Colombet M, Stiller CA, Pritchard-Jones K; Steliarova-Foucher E; IICC-3 Contributors. Incidence of childhood renal tumours: an international population-based study. Int J Cancer. 2020; 147:3313–27.4. Ray S, Jones R, Pritchard-Jones K, Dzhuma K, van den Heuvel-Eibrink M, Tytgat G, et al. Pediatric and young adult renal cell carcinoma. Pediatr Blood Cancer. 2020; 67:e28675.5. Nakata K, Williams R, Kinoshita Y, Koshinaga T, Moroz V, Al-Saadi R, et al. Comparative analysis of the clinical characteristics and outcomes of patients with Wilms tumor in the United Kingdom and Japan. Pediatr Blood Cancer. 2021; 68:e29143.6. Loke BN, Wong MK, Tawng KD, Kuick CH, Jain S, Lian D, et al. Clinical, pathological and loss of heterozygosity differences in Wilms tumors between Asian and non-Asian children. Int J Cancer. 2019; 144:1234–42.7. Ooms A, Vujanic GM, D’Hooghe E, Collini P, L’Hermine-Coulomb A, Vokuhl C, et al. Renal tumors of childhood: a histopathologic pattern-based diagnostic approach. Cancers (Basel). 2020; 12:729.8. Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006; 43:705–15.9. Liu EK, Suson KD. Syndromic Wilms tumor: a review of predisposing conditions, surveillance and treatment. Transl Androl Urol. 2020; 9:2370–81.10. Jain J, Sutton KS, Hong AL. Progress update in pediatric renal tumors. Curr Oncol Rep. 2021; 23:33.11. Oue T, Fukuzawa M, Koshinaga T, Okita H, Nozaki M, Chin M, et al. Management of pediatric renal tumor: past and future trials of the Japan Wilms Tumor Study Group. Pediatr Int. 2015; 57:828–31.12. Dome JS, Graf N, Geller JI, Fernandez CV, Mullen EA, Spreafico F, et al. Advances in Wilms tumor treatment and biology: progress through international collaboration. J Clin Oncol. 2015; 33:2999–3007.13. Pater L, Melchior P, Rube C, Cooper BT, McAleer MF, Kalapurakal JA, et al. Wilms tumor. Pediatr Blood Cancer. 2021; 68(Suppl 2):e28257.14. Aldrink JH, Heaton TE, Dasgupta R, Lautz TB, Malek MM, Abdessalam SF, et al. Update on Wilms tumor. J Pediatr Surg. 2019; 54:390–7.15. Suh WS, Kang IJ, Koo HH, Kook H, Kim SK, Kim HK, et al. Epidemiology and clinical outcomes of childhood Wilms tumor in Korea. Korean J Pediatr Hematol Oncol. 204. 11:164–70.16. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016; 48:869–82.17. Ahmed HU, Arya M, Levitt G, Duffy PG, Mushtaq I, Sebire NJ. Part I: Primary malignant non-Wilms’ renal tumours in children. Lancet Oncol. 2007; 8:730–7.18. van den Heuvel-Eibrink MM, van Tinteren H, Rehorst H, Coulombe A, Patte C, de Camargo B, et al. Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr Blood Cancer. 2011; 56:733–7.19. Furtwangler R, Gooskens SL, van Tinteren H, de Kraker J, Schleiermacher G, Bergeron C, et al. Clear cell sarcomas of the kidney registered on International Society of Pediatric Oncology (SIOP) 93-01 and SIOP 2001 protocols: a report of the SIOP Renal Tumour Study Group. Eur J Cancer. 2013; 49:3497–506.20. Park JE, Noh OK, Lee Y, Choi HS, Han JW, Hahn SM, et al. Loss of heterozygosity at chromosome 16q is a negative prognostic factor in Korean pediatric patients with favorable histology Wilms tumor: a report of the Korean Pediatric Hematology Oncology Group (K-PHOG). Cancer Res Treat. 2020; 52:438–45.21. Vujanic GM, Gessler M, Ooms A, Collini P, Coulomb-l’Hermine A, D’Hooghe E, et al. The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat Rev Urol. 2018; 15:693–701.22. de la Monneraye Y, Michon J, Pacquement H, Aerts I, Orbach D, Doz F, et al. Indications and results of diagnostic biopsy in pediatric renal tumors: a retrospective analysis of 317 patients with critical review of SIOP guidelines. Pediatr Blood Cancer. 2019; 66:e27641.23. Rossoff J, Tse WT, Duerst RE, Schneiderman J, Morgan E, Kletzel M, et al. High-dose chemotherapy and autologous hematopoietic stem-cell rescue for treatment of relapsed and refractory Wilms tumor: re-evaluating outcomes. Pediatr Hematol Oncol. 2018; 35:316–21.24. Qureshi SS, Bhagat M, Verma K, Yadav S, Prasad M, Vora T, et al. Incidence, treatment, and outcomes of primary and recurrent Non-Wilms renal tumors in children: report of 109 patients treated at a single institution. J Pediatr Urol. 2020; 16:475.25. Saula PW, Hadley GP. Pediatric non-Wilms’ renal tumors: a third world experience. World J Surg. 2012; 36:565–72.26. Geller JI, Cost NG, Chi YY, Tornwall B, Cajaiba M, Perlman EJ, et al. A prospective study of pediatric and adolescent renal cell carcinoma: a report from the Children’s Oncology Group AREN0321 study. Cancer. 2020; 126:5156–64.27. Seibel NL, Chi YY, Perlman EJ, Tian J, Sun J, Anderson JR, et al. Impact of cyclophosphamide and etoposide on outcome of clear cell sarcoma of the kidney treated on the National Wilms Tumor Study-5 (NWTS-5). Pediatr Blood Cancer. 2019; 66:e27450.28. Tomlinson GE, Breslow NE, Dome J, Guthrie KA, Norkool P, Li S, et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005; 23:7641–5.29. Gooskens SL, Houwing ME, Vujanic GM, Dome JS, Diertens T, Coulomb-l’Hermine A, et al. Congenital mesoblastic nephroma 50 years after its recognition: a narrative review. Pediatr Blood Cancer. 2017; 64:e26437.30. Ahmed HU, Arya M, Levitt G, Duffy PG, Sebire NJ, Mushtaq I. Part II: Treatment of primary malignant non-Wilms’ renal tumours in children. Lancet Oncol. 2007; 8:842–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neutropenia following metamizole use in pediatric patients: a multicenter retrospective study
- Korean Society for Pediatric Neuro-Oncology Protocol for Germ Cell Tumors
- Renal Tumors in Children
- Immunotherapy in Pediatric Solid Tumors
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms