Cancer Res Treat.
2023 Jan;55(1):123-135. 10.4143/crt.2021.1561.
Impacts of Subtype on Clinical Feature and Outcome of Male Breast Cancer: Multicenter Study in Korea (KCSG BR16-09)
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, College of Medicine, Yonsei University, Seoul, Korea
- 4Division of Oncology-Hematology, Department of Internal Medicine, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea
- 5Department of Surgery, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea
- 6Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Division of Medical Oncology, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 8Department of Internal Medicine, Ulsan University Hospital, Ulsan University College of Medicine, Ulsan, Korea
- 9Department of Hematology and Oncology, Ewha Womans University Hospital, Seoul, Korea
- 10Department of Hemato-Oncology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 11Division of Hematology‐Oncology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 12Division of Medical Oncology, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 13Division of Medical Oncology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 14Division of Medical Oncology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 15Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 16Division of Hematology and Oncology, Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea
- 17Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 18Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 19Division of Medical Oncology, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2537998
- DOI: http://doi.org/10.4143/crt.2021.1561
Abstract
- Purpose
The treatment of male breast cancer (MBC) has been extrapolated from female breast cancer (FBC) because of its rarity despite their different clinicopathologic characteristics. We aimed to investigate the distribution of intrinsic subtypes based on immunohistochemistry, their clinical impact, and treatment pattern in clinical practice through a multicenter study in Korea.
Materials and Methods
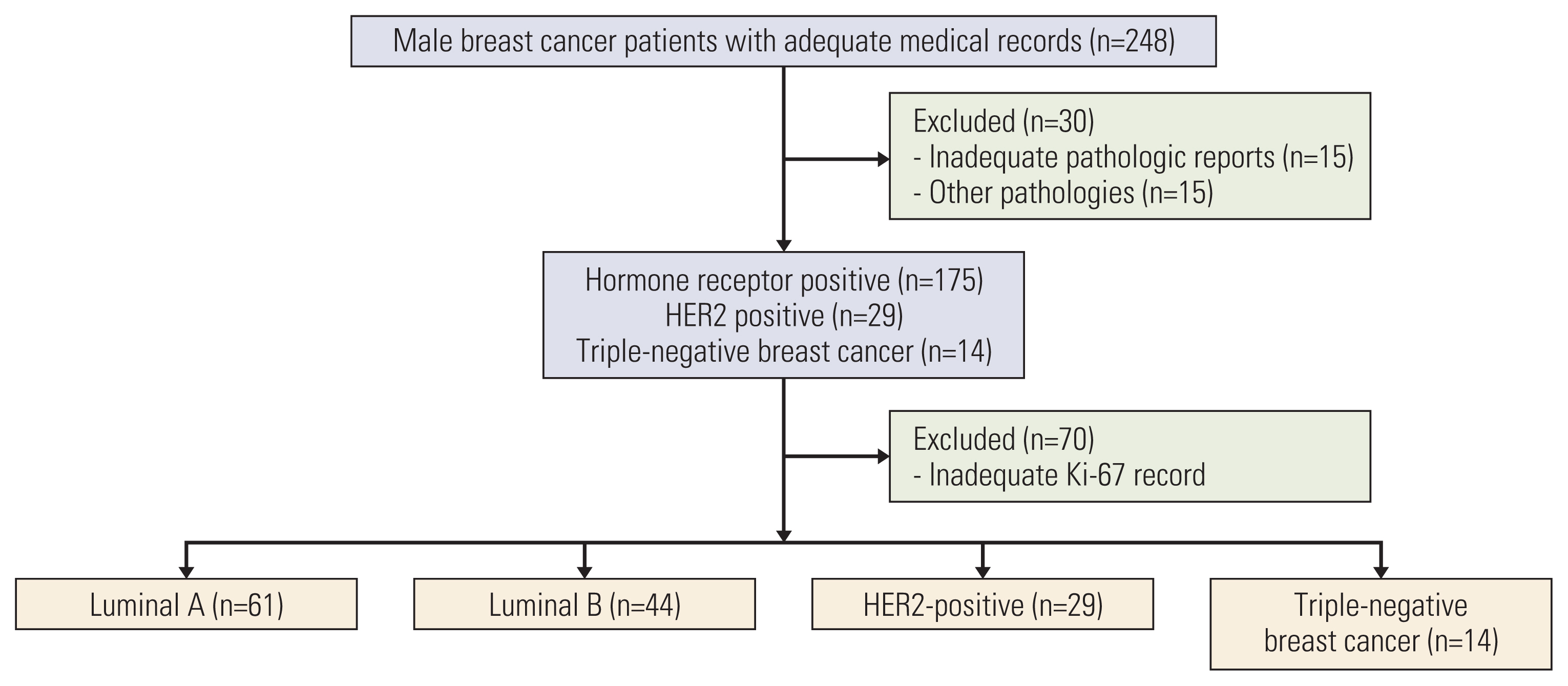
We retrospectively analyzed clinical data of 248 MBC patients from 18 institutions across the country from January 1995 to July 2016.
Results
The median age of MBC patients was 63 years (range, 25 to 102 years). Among 148 intrinsic subtype classified patients, 61 (41.2%), 44 (29.7%), 29 (19.5%), and 14 (9.5%) were luminal A, luminal B, human epidermal growth factor receptor 2, and triple-negative breast cancer, respectively. Luminal A subtype showed trends for superior survival compared to other subtypes. Most hormone receptor-positive patients (166 patients, 82.6%) received adjuvant endocrine treatment. Five-year completion of adjuvant endocrine treatment was associated with superior disease-free survival (DFS) in patients classified with an intrinsic subtype (hazard ratio [HR], 0.15; 95% confidence interval [CI], 0.04 to 0.49; p=0.002) and in all patients (HR, 0.16; 95% CI, 0.05 to 0.54; p=0.003).
Conclusion
Distribution of subtypes of MBC was similar to FBC and luminal type A was most common. Overall survival tended to be improved for luminal A subtype, although there was no statistical significance. Completion of adjuvant endocrine treatment was associated with prolonged DFS in intrinsic subtype classified patients. MBC patients tended to receive less treatment. MBC patients should receive standard treatment according to guidelines as FBC patients.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.2. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021; 53:301–15.3. Lee EG, Jung SY, Lim MC, Lim J, Kang HS, Lee S, et al. Comparing the characteristics and outcomes of male and female breast cancer patients in Korea: Korea Central Cancer Registry. Cancer Res Treat. 2020; 52:739–46.4. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute;2018.5. Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017; 44:267–72.6. Giordano SH. Breast cancer in men. N Engl J Med. 2018; 378:2311–20.7. Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, et al. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002; 4:R2.8. Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat. 2011; 126:771–8.9. Friedman LS, Gayther SA, Kurosaki T, Gordon D, Noble B, Casey G, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997; 60:313–9.10. Ottini L, Masala G, D’Amico C, Mancini B, Saieva C, Aceto G, et al. BRCA1 and BRCA2 mutation status and tumor characteristics in male breast cancer: a population-based study in Italy. Cancer Res. 2003; 63:342–7.11. Cardoso F, Bartlett JM, Slaets L, van Deurzen CH, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018; 29:405–17.12. Piscuoglio S, Ng CK, Murray MP, Guerini-Rocco E, Martelotto LG, Geyer FC, et al. The genomic landscape of male breast cancers. Clin Cancer Res. 2016; 22:4045–56.13. Callari M, Cappelletti V, De Cecco L, Musella V, Miodini P, Veneroni S, et al. Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat. 2011; 127:601–10.14. Shaaban AM, Ball GR, Brannan RA, Cserni G, Di Benedetto A, Dent J, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012; 133:949–58.15. Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020; 38:1849–63.16. Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010; 28:2114–22.17. Hong JH, Ha KS, Jung YH, Won HS, An HJ, Lee GJ, et al. Clinical features of male breast cancer: experiences from seven institutions over 20 years. Cancer Res Treat. 2016; 48:1389–98.18. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010; 134:e48–72.19. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131:18–43.20. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.21. Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care (Basel). 2015; 10:124–30.22. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 7th ed. New York: Springer;2010.23. Korean Breast Cancer Society. Breast Cancer Facts and Figures 2020. Seoul: Korean Breast Cancer Society;2020.24. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012; 366:520–9.25. Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. 2020; 180:471–9.26. Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020; 126:26–36.27. Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, Laronningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011; 29:4381–6.28. Johansson I, Ringner M, Hedenfalk I. The landscape of candidate driver genes differs between male and female breast cancer. PLoS One. 2013; 8:e78299.29. Oke O, Niu J, Chavez-MacGregor M, Zhao H, Giordano SH. Adjuvant tamoxifen adherence in men with early-stage breast cancer. Cancer. 2022; 128:59–64.30. Gnerlich JL, Deshpande AD, Jeffe DB, Seelam S, Kimbuende E, Margenthaler JA. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011; 18:1837–44.31. Marchal F, Salou M, Marchal C, Lesur A, Desandes E. Men with breast cancer have same disease-specific and event-free survival as women. Ann Surg Oncol. 2009; 16:972–8.32. Grenader T, Yerushalmi R, Tokar M, Fried G, Kaufman B, Peretz T, et al. The 21-gene recurrence score assay (Oncotype DX) in estrogen receptor-positive male breast cancer: experience in an Israeli cohort. Oncology. 2014; 87:1–6.33. Massarweh SA, Sledge GW, Miller DP, McCullough D, Petkov VI, Shak S. Molecular characterization and mortality from breast cancer in men. J Clin Oncol. 2018; 36:1396–404.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- A Review on Gynecomastia and Male Breast Cancer for Radiologists
- Sonographic Features of Palpable Breast and Axillary Lesions in Adult Male Patients: A Pictorial Essay
- The Survival and Financial Benefit of Investigator-Initiated Trials Conducted by Korean Cancer Study Group
- Breast Cancer During Pregnancy