Endocrinol Metab.
2022 Dec;37(6):891-900. 10.3803/EnM.2022.1590.
Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
- Affiliations
-
- 1Laboratory of Endocrinology and Immune System, Chungnam National University College of Medicine, Daejeon, Korea
- 2Department of Medical Science, Chungnam National University College of Medicine, Daejeon, Korea
- 3Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 4Department of Biochemistry, Chungnam National University College of Natural Sciences, Daejeon, Korea
- 5Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan
- KMID: 2537290
- DOI: http://doi.org/10.3803/EnM.2022.1590
Abstract
- Background
An excess of thyroid hormones in Graves’ disease (GD) has profound effects on systemic energy metabolism that are currently partially understood. In this study, we aimed to provide a comprehensive understanding of the metabolite changes that occur when patients with GD transition from hyperthyroidism to euthyroidism with methimazole treatment.
Methods
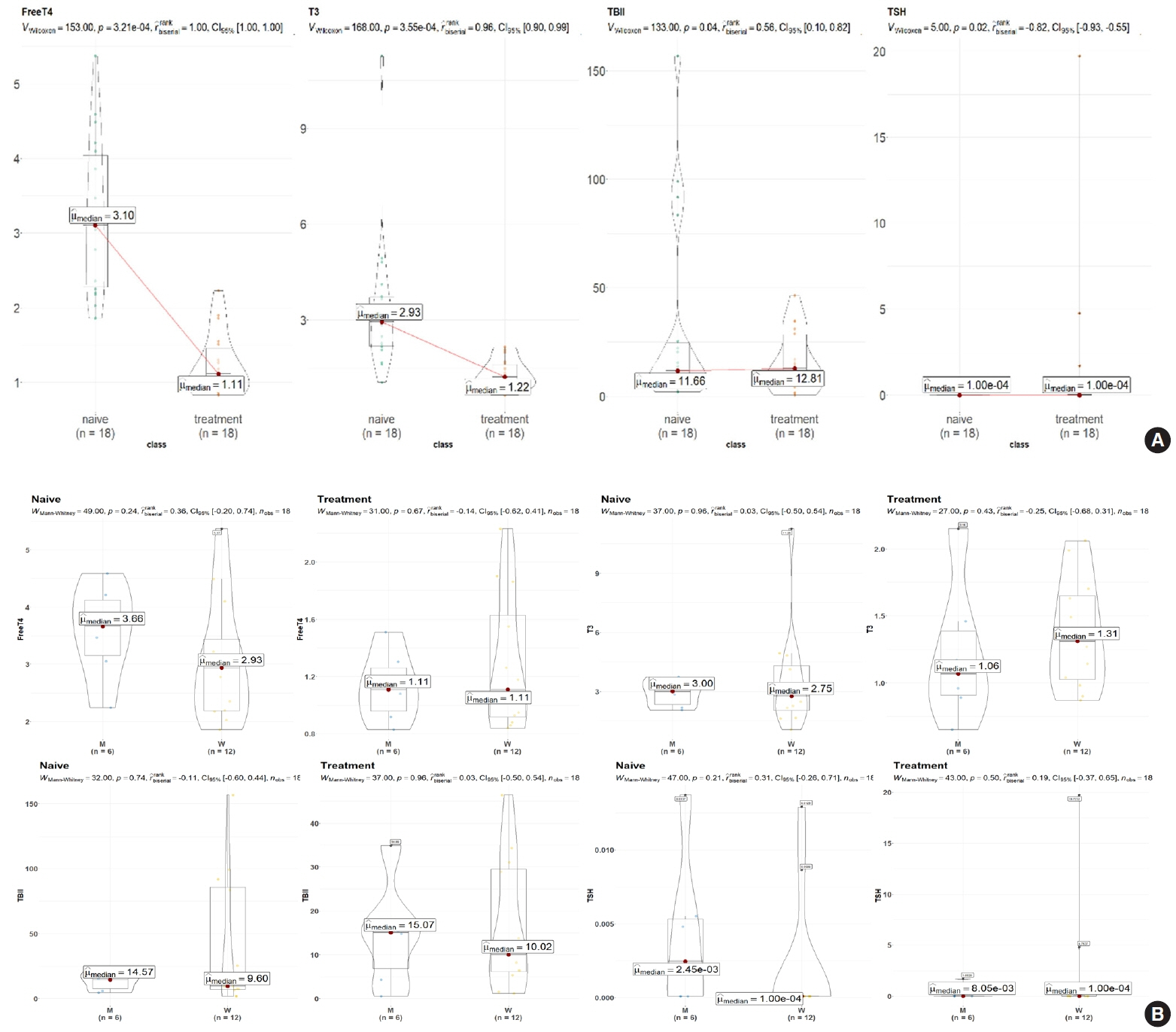
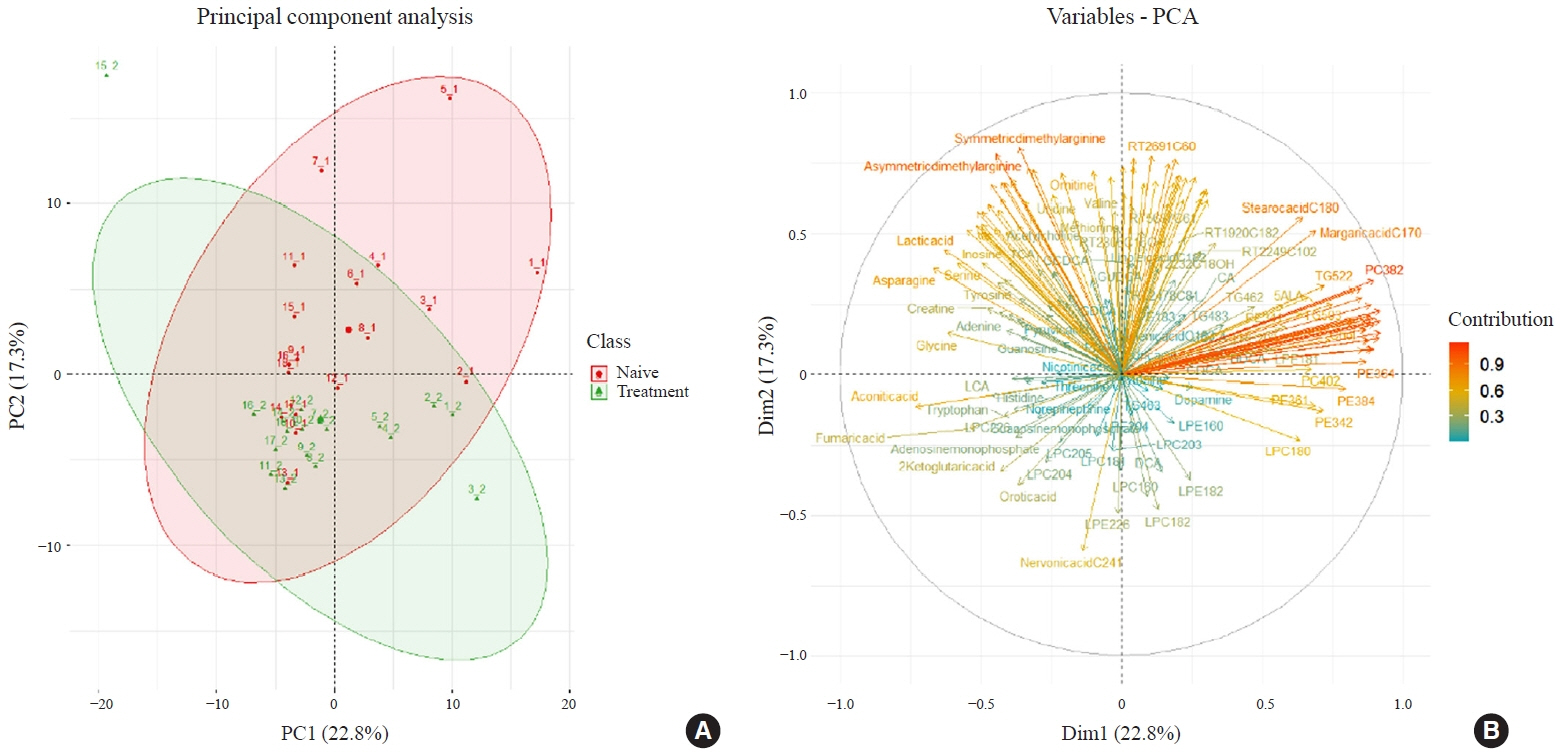
Eighteen patients (mean age, 38.6±14.7 years; 66.7% female) with newly diagnosed or relapsed GD attending the endocrinology outpatient clinics in a single institution were recruited between January 2019 and July 2020. All subjects were treated with methimazole to achieve euthyroidism. We explored metabolomics by performing liquid chromatography-mass spectrometry analysis of plasma samples of these patients and then performed multivariate statistical analysis of the metabolomics data.
Results
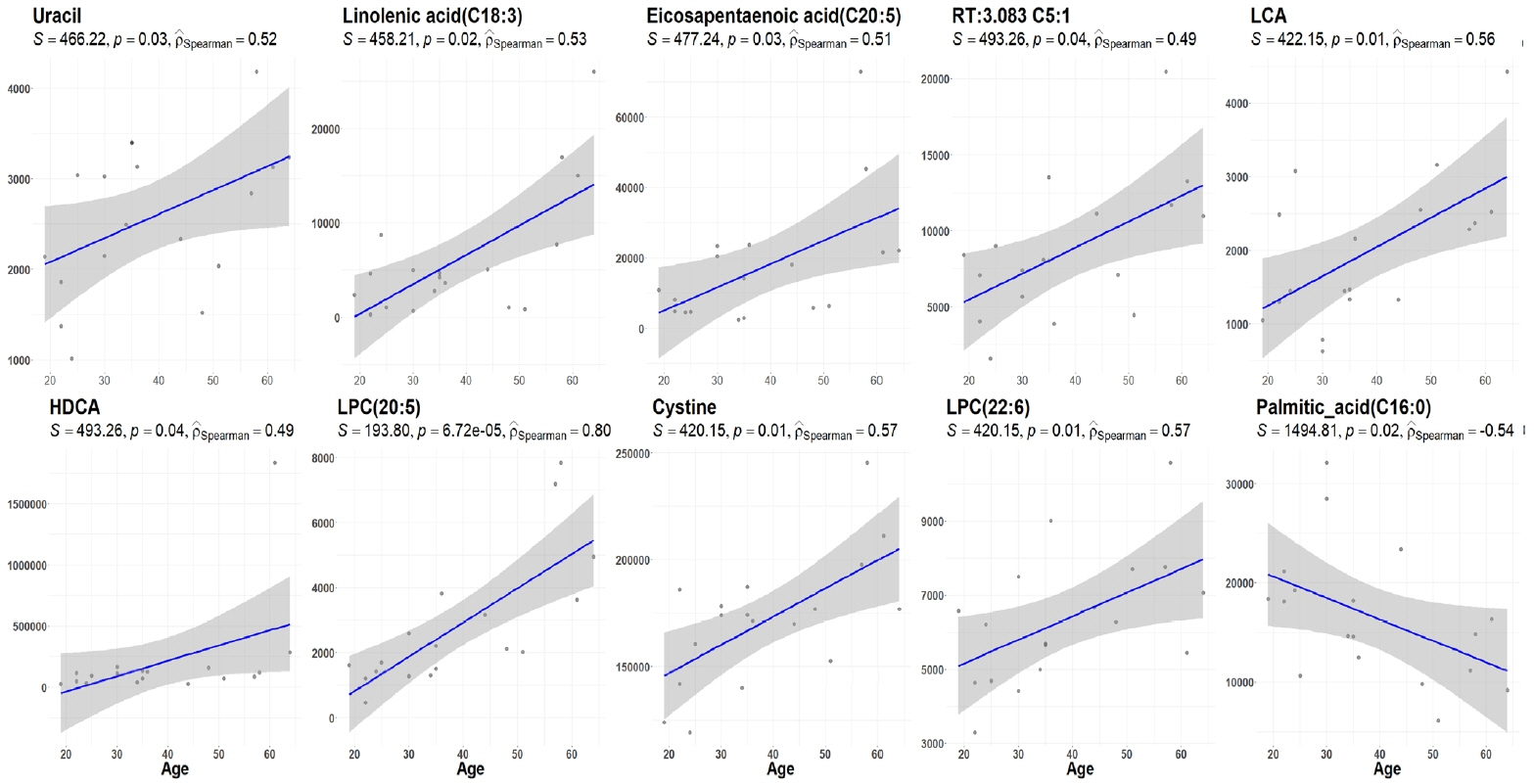
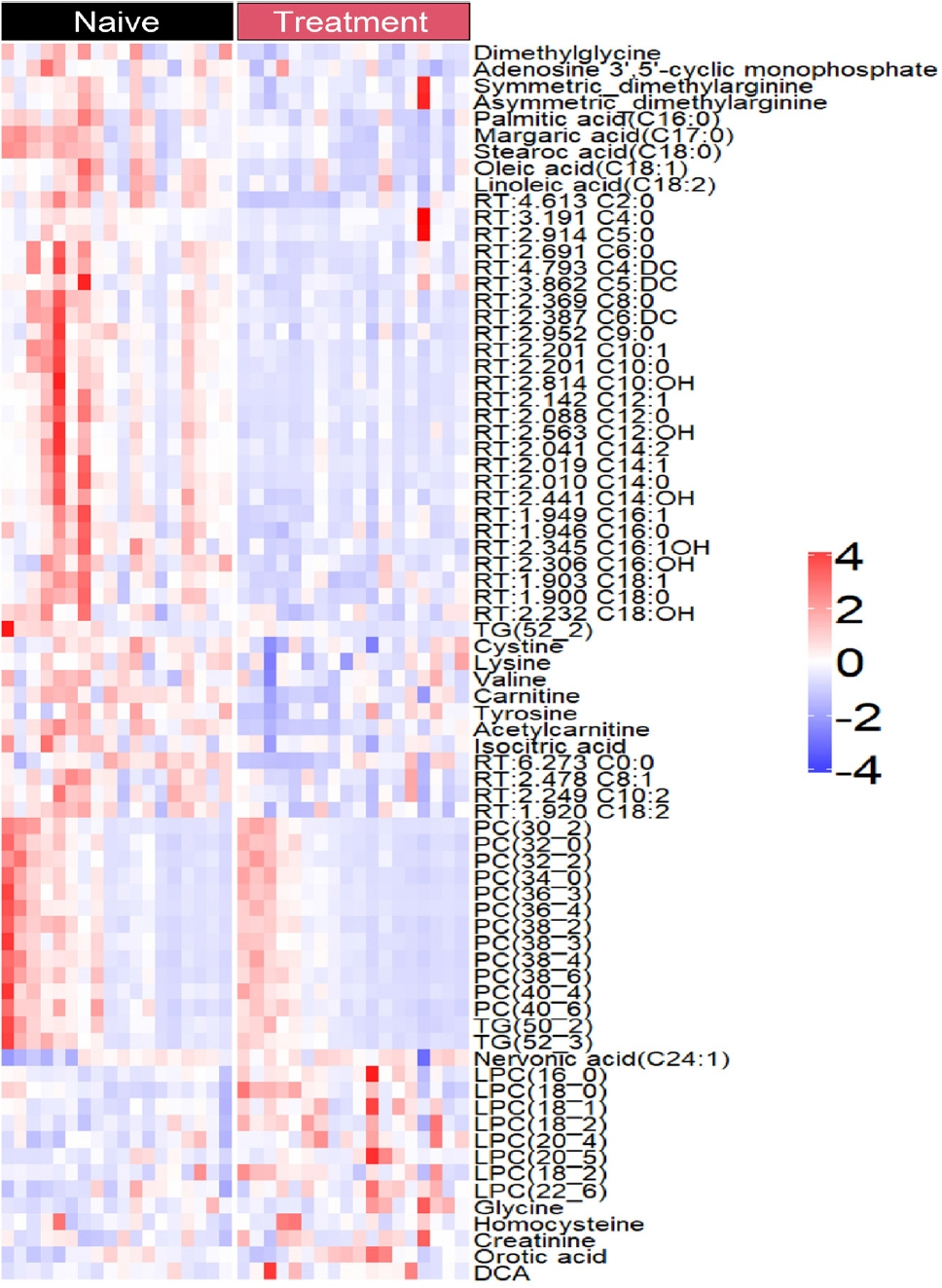
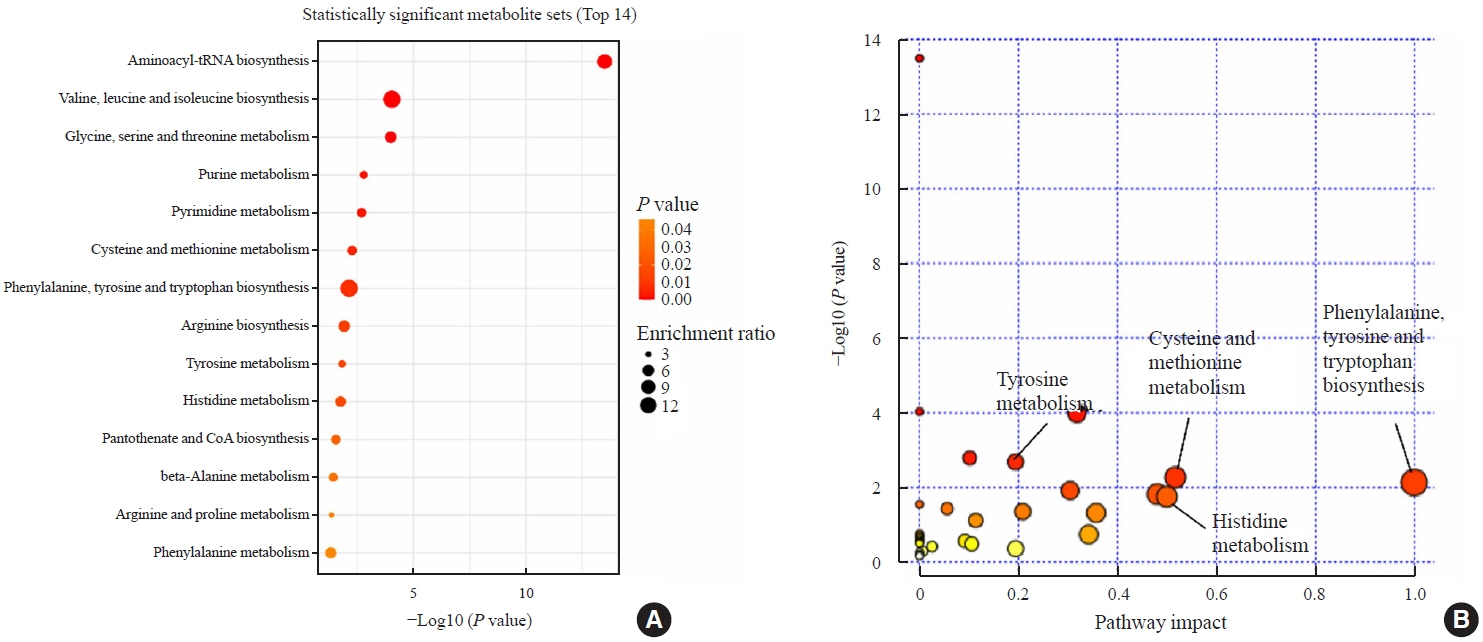
Two hundred metabolites were measured before and after 12 weeks of methimazole treatment in patients with GD. The levels of 61 metabolites, including palmitic acid (C16:0) and oleic acid (C18:1), were elevated in methimazole-naïve patients with GD, and these levels were decreased by methimazole treatment. The levels of another 15 metabolites, including glycine and creatinine, were increased after recovery of euthyroidism upon methimazole treatment in patients with GD. Pathway analysis of metabolomics data showed that hyperthyroidism was closely related to aminoacyl-transfer ribonucleic acid biosynthesis and branched-chain amino acid biosynthesis pathways.
Conclusion
In this study, significant variations of plasma metabolomic patterns that occur during the transition from hyperthyroidism to euthyroidism were detected in patients with GD via untargeted metabolomics analysis.
Keyword
Figure
Reference
-
1. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008; 18:141–4.
Article2. Greenlund LJ, Nair KS, Brennan MD. Changes in body composition in women following treatment of overt and subclinical hyperthyroidism. Endocr Pract. 2008; 14:973–8.
Article3. Lee JC, Song BS, Kang YM, Kim YR, Kang YE, Lee JH, et al. Effect of thyroid-stimulating hormone suppression on muscle function after total thyroidectomy in patients with thyroid cancer. Front Endocrinol (Lausanne). 2021; 12:769074.
Article4. Brent GA. Clinical practice: Graves’ disease. N Engl J Med. 2008; 358:2594–605.5. Song E, Koo MJ, Noh E, Hwang SY, Park MJ, Kim JA, et al. Risk of diabetes in patients with long-standing Graves’ disease: a longitudinal study. Endocrinol Metab (Seoul). 2021; 36:1277–86.
Article6. Eom YS, Wilson JR, Bernet VJ. Links between thyroid disorders and glucose homeostasis. Diabetes Metab J. 2022; 46:239–56.7. Mynatt RL, Park EA, Thorngate FE, Das HK, Cook GA. Changes in carnitine palmitoyltransferase-I mRNA abundance produced by hyperthyroidism and hypothyroidism parallel changes in activity. Biochem Biophys Res Commun. 1994; 201:932–7.8. Goglia F, Moreno M, Lanni A. Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Lett. 1999; 452:115–20.
Article9. Al-Majdoub M, Lantz M, Spegel P. Treatment of Swedish patients with Graves’ hyperthyroidism is associated with changes in acylcarnitine levels. Thyroid. 2017; 27:1109–17.
Article10. Song J, Shan Z, Mao J, Teng W. Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinol (Oxf). 2019; 90:727–36.
Article11. Liu J, Fu J, Jia Y, Yang N, Li J, Wang G. Serum metabolomic patterns in patients with autoimmune thyroid disease. Endocr Pract. 2020; 26:82–96.
Article12. Xia Q, Qian W, Chen L, Chen X, Xie R, Zhang D, et al. Comprehensive metabolomics study in children with Graves’ disease. Front Endocrinol (Lausanne). 2021; 12:752496.
Article13. Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008; 11:45–9.
Article14. Setoyama D, Lee HY, Moon JS, Tian J, Kang YE, Lee JH, et al. Immunometabolic signatures predict recovery from thyrotoxic myopathy in patients with Graves’ disease. J Cachexia Sarcopenia Muscle. 2022; 13:355–67.
Article15. Srivastava S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites. 2019; 9:301.
Article16. Mariash CN. Thyroid hormone and the adipocyte. J Clin Endocrinol Metab. 2003; 88:5603–4.
Article17. Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018; 14:259–69.
Article18. Mendelson SD. Metabolic syndrome and psychiatric illness: interactions, pathophysiology, assessment and treatment. Boston: Elsevier/Academic Press;2008.19. Jurand J, Oliver MF. Effect of thyroid activity on fatty acid composition of serum lipids. Atherosclerosis. 1970; 11:125–40.
Article20. Zhou G, Xu Y, Zhai Y, Gong Z, Xu K, Wang G, et al. The association between serum palmitic acid and thyroid function. Front Endocrinol (Lausanne). 2022; 13:860634.
Article21. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012; 97:1554–62.
Article22. Chen X, Zheng X, Ding Z, Su Y, Wang S, Cui B, et al. Relationship of gender and age on thyroid hormone parameters in a large Chinese population. Arch Endocrinol Metab. 2020; 64:52–8.
Article23. Hoogendoorn EH, Hermus AR, de Vegt F, Ross HA, Verbeek AL, Kiemeney LA, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem. 2006; 52:104–11.
Article24. Nelson DL, Cox MM. Principles of biochemistry. 4th ed. New York: Freeman;2004.25. Ntountoumi C, Vlastaridis P, Mossialos D, Stathopoulos C, Iliopoulos I, Promponas V, et al. Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved. Nucleic Acids Res. 2019; 47:9998–10009.
Article26. Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, et al. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. 2015; 14:67.
Article27. Holecek M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond). 2018; 15:33.
Article28. Krishnamurthy HK, Reddy S, Jayaraman V, Krishna K, Song Q, Rajasekaran KE, et al. Effect of micronutrients on thyroid parameters. J Thyroid Res. 2021; 2021:1865483.
Article29. Louard RJ, Barrett EJ, Gelfand RA. Overnight branchedchain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism. 1995; 44:424–9.
Article30. Sun L, Goh HJ, Verma S, Govindharajulu P, Sadananthan SA, Michael N, et al. Brown adipose tissues mediate the metabolism of branched chain amino acids during the transitioning from hyperthyroidism to euthyroidism (TRIBUTE). Sci Rep. 2022; 12:3693.
Article31. Mannisto PT, Mattila J, Tuominen RK, Vesalainen S. Effects of some putative amino acid neurotransmitters on the stimulated TSH secretion in male rats. Horm Res. 1983; 17:19–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pulmonary Arterial Hypertension Associated With Hyperthyroidism, Persistent After Euthyroidism Was Obtained
- Isolated Pulmonary Arterial Hypertension-Janus' Faces of Hyperthyroidism
- Appropriate Surgical Extent in the Surgery for Graves' Disease
- Euthyroid Graves' Ophthalmopathy with Negative Autoantibodies
- The Diagnosis and Management of Hyperthyroidism in Korea: Consensus Report of the Korean Thyroid Association