Intest Res.
2022 Oct;20(4):392-417. 10.5217/ir.2021.00160.
Involvement of the cannabinoid system in chronic inflammatory intestinal diseases: opportunities for new therapies
- Affiliations
-
- 1Department of Pharmacology, Institute of Biological Sciences (ICB), Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil
- 2Graduate Program in Physiology and Pharmacology, Institute of Biological Sciences (ICB), Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil
- 3Department of Pharmacology, Institute of Biological Sciences (ICB), Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil
- KMID: 2535815
- DOI: http://doi.org/10.5217/ir.2021.00160
Abstract
- The components of the endogenous cannabinoid system are widely expressed in the gastrointestinal tract contributing to local homeostasis. In general, cannabinoids exert inhibitory actions in the gastrointestinal tract, inducing anti-inflammatory, antiemetic, antisecretory, and antiproliferative effects. Therefore, cannabinoids are interesting pharmacological compounds for the treatment of several acute intestinal disorders, such as dysmotility, emesis, and abdominal pain. Likewise, the role of cannabinoids in the treatment of chronic intestinal diseases, such as irritable bowel syndrome and inflammatory bowel disease, is also under investigation. Patients with chronic intestinal inflammatory diseases present impaired quality of life, and mental health issues are commonly associated with long-term chronic diseases. The complex pathophysiology of these diseases contributes to difficulties in diagnosis and, therefore, in the choice of a satisfactory treatment. Thus, this article reviews the involvement of the cannabinoid system in chronic inflammatory diseases that affect the gastrointestinal tract and highlights possible therapeutic approaches related to the use of cannabinoids.
Keyword
Figure
Reference
-
1. González-Mariscal I, Krzysik-Walker SM, Doyle ME, et al. Human CB1 receptor isoforms, present in hepatocytes and β-cells, are involved in regulating metabolism. Sci Rep. 2016; 6:33302.
Article2. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018; 19:833.
Article3. Zhang HY, Bi GH, Li X, et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology. 2015; 40:1037–1051.
Article4. Liu QR, Pan CH, Hishimoto A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009; 8:519–530.
Article5. Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008; 153:277–285.6. Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol. 2007; 72:1393–1401.
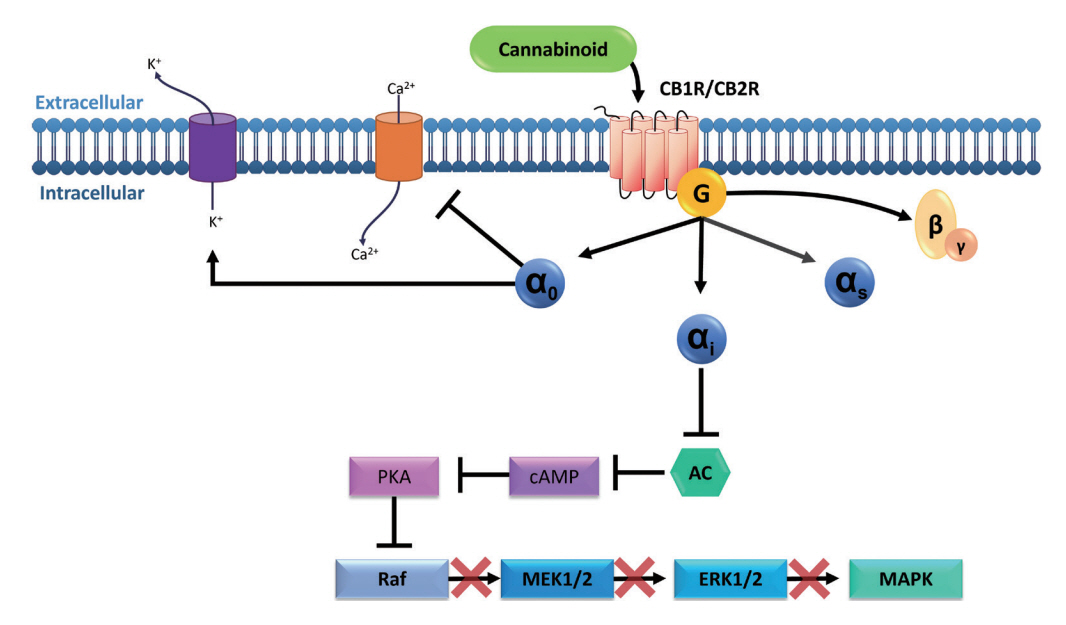
Article7. Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol. 2010; 44:75–85.
Article8. Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond). 2006; 30 Suppl 1:S13–S18.
Article9. Brunton LL, Hilal-Dandan R, Knollmann BC. As Bases Farmacológicas da Terapêutica de Goodman and Gilman. 13rd ed. Porto Alegre: Artmed Editora;2018.10. Blackwood BP, Wood DR, Yuan C, et al. A role for cAMP and protein kinase A in experimental necrotizing enterocolitis. Am J Pathol. 2017; 187:401–417.
Article11. Börner C, Smida M, Höllt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009; 284:35450–35460.
Article12. Liu YJ, Fan HB, Jin Y, et al. Cannabinoid receptor 2 suppresses leukocyte inflammatory migration by modulating the JNK/c-Jun/Alox5 pathway. J Biol Chem. 2013; 288:13551–13562.
Article13. Cencioni MT, Chiurchiù V, Catanzaro G, et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One. 2010; 5:e8688.
Article14. Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015; 36:277–296.
Article15. Huang XL, Xu J, Zhang XH, et al. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm Res. 2011; 60:727–734.
Article16. Chen Q, Duan X, Fan H, et al. Oxymatrine protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway. Int Immunopharmacol. 2017; 53:149–157.
Article17. Zhang D, Wang L, Yan L, et al. Vacuolar protein sorting 4B regulates apoptosis of intestinal epithelial cells via p38 MAPK in Crohn’s disease. Exp Mol Pathol. 2015; 98:55–64.
Article18. Miguel JC, Maxwell AA, Hsieh JJ, et al. Epidermal growth factor suppresses intestinal epithelial cell shedding through a MAPK-dependent pathway. J Cell Sci. 2017; 130:90–96.19. Docena G, Rovedatti L, Kruidenier L, et al. Down-regulation of p38 mitogen-activated protein kinase activation and proinflammatory cytokine production by mitogen-activated protein kinase inhibitors in inflammatory bowel disease. Clin Exp Immunol. 2010; 162:108–115.
Article20. Gao W, Wang C, Yu L, et al. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed Res Int. 2019; 2019:6769789.
Article21. Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011; 51:26–38.
Article22. Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012; 35:529–558.
Article23. Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005; (168):1–51.
Article24. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015; 12:692–698.
Article25. Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocytetype 12-lipoxygenase. Biochemistry. 2001; 40:861–866.
Article26. Kozak KR, Crews BC, Morrow JD, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002; 277:44877–44885.
Article27. Petrie JR, Vanhercke T, Shrestha P, et al. Recruiting a new substrate for triacylglycerol synthesis in plants: the monoacylglycerol acyltransferase pathway. PLoS One. 2012; 7:e35214.
Article28. Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology. 2016; 151:252–266.
Article29. Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008; 153:263–270.
Article30. Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002; 54:161–202.
Article31. Horvath TL. Endocannabinoids and the regulation of body fat: the smoke is clearing. J Clin Invest. 2003; 112:323–326.
Article32. Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008; 20:850–857.
Article33. Miller LK, Devi LA. The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications. Pharmacol Rev. 2011; 63:461–470.
Article34. Borowska M, Czarnywojtek A, Sawicka-Gutaj N, et al. The effects of cannabinoids on the endocrine system. Endokrynol Pol. 2018; 69:705–719.
Article35. Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL. Cannabinoid receptor 2 (CB2) signals via G-alpha-s and Induces IL-6 and IL-10 cytokine secretion in human primary leukocytes. ACS Pharmacol Transl Sci. 2019; 2:414–428.
Article36. Fernández-López D, Lizasoain I, Moro MA, Martínez-Orgado J. Cannabinoids: well-suited candidates for the treatment of perinatal brain injury. Brain Sci. 2013; 3:1043–1059.
Article37. Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008; 68:339–342.
Article38. Dhopeshwarkar A, Mackie K. CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol Pharmacol. 2014; 86:430–437.
Article39. Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003; 27:289–301.
Article40. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998; 92:573–585.
Article41. Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetiterelated neural circuit. Neuron. 2005; 48:1055–1066.
Article42. Gamber KM, Macarthur H, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005; 49:646–652.
Article43. Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008; 3:e1797.
Article44. DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011; 108:12904–12908.
Article45. DiPatrizio NV, Igarashi M, Narayanaswami V, et al. Fasting stimulates 2-AG biosynthesis in the small intestine: role of cholinergic pathways. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R805–13.
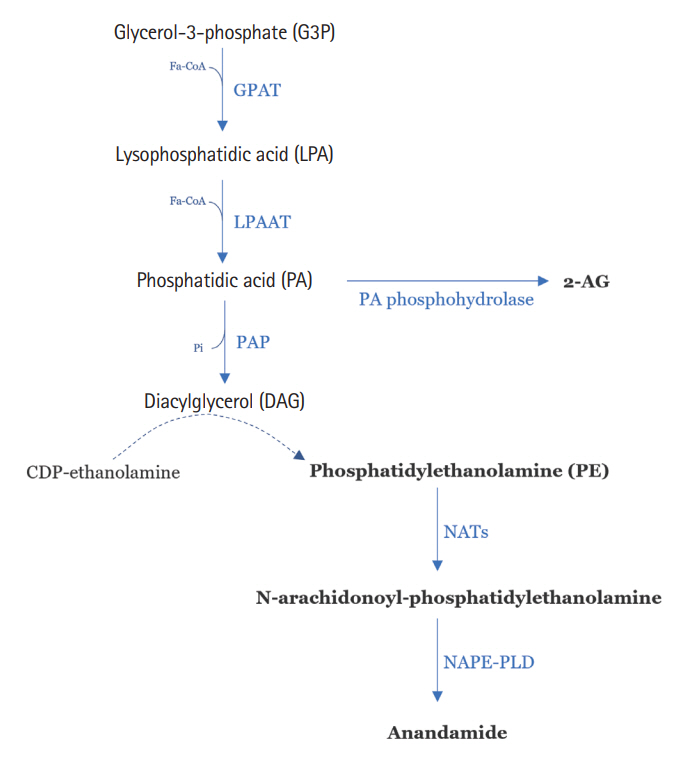
Article46. Bozelli JC Jr, Epand RM. Specificity of acyl chain composition of phosphatidylinositols. Proteomics. 2019; 19:e1900138.
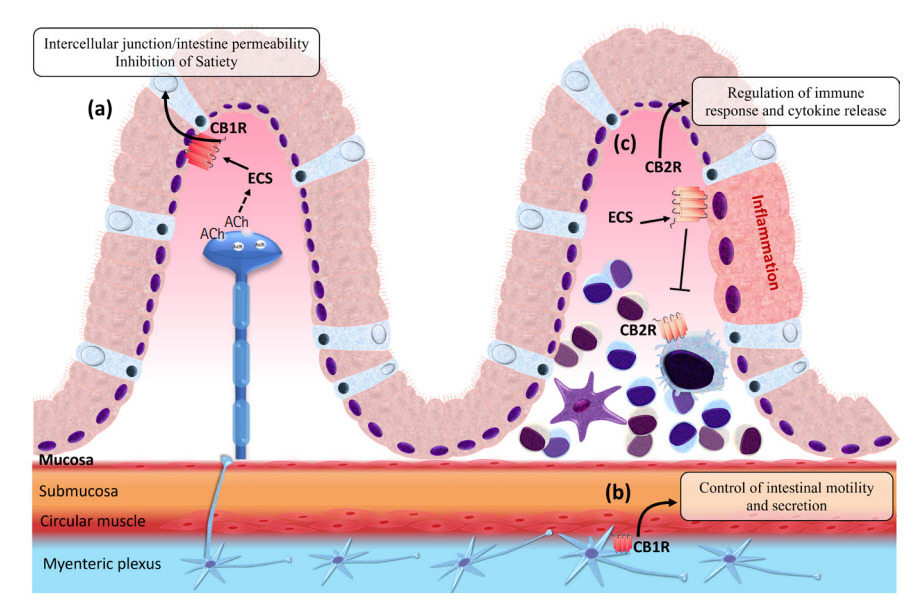
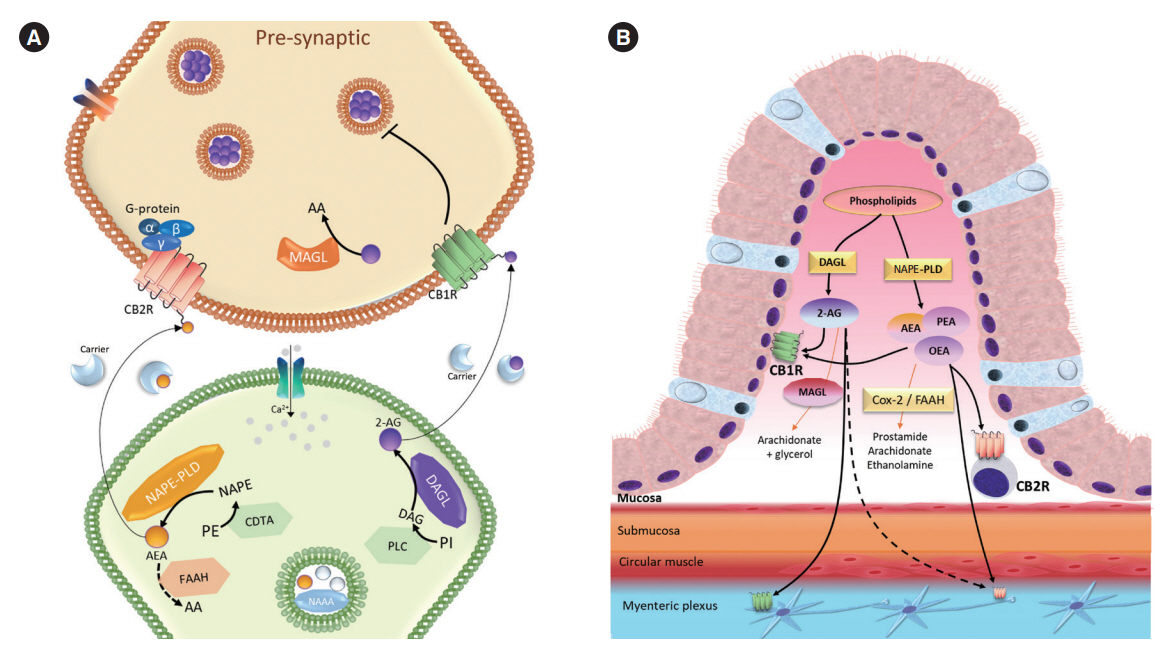
Article47. Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005; 22:667–683.
Article48. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012; 9:286–294.
Article49. Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997; 121:1557–1566.
Article50. Kulkarni-Narla A, Brown DR. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000; 302:73–80.
Article51. Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol. 2002; 448:410–422.
Article52. Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004; 142:1247–1254.
Article53. Abalo R, Vera G, López-Pérez AE, Martínez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012; 90:1–10.
Article54. DiPatrizio NV. Endocannabinoids in the gut. Cannabis Cannabinoid Res. 2016; 1:67–77.
Article55. Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001; 48:859–867.
Article56. Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001; 81:715–723.
Article57. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005; 5:400–411.
Article58. Greineisen WE, Turner H. Immunoactive effects of cannabinoids: considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol. 2010; 10:547–555.
Article59. Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011; 4:574–583.
Article60. Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006; 55:1373–1376.
Article61. Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004; 113:1202–1209.
Article62. Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005; 129:437–453.
Article63. Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006; 18:949–956.
Article64. Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007; 13:35–37.
Article65. Ihenetu K, Molleman A, Parsons ME, Whelan CJ. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003; 458:207–215.
Article66. Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014; 722:134–146.
Article67. Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005; (168):573–598.
Article68. Storr MA, Sharkey KA. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol. 2007; 7:575–582.
Article69. Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005; 310:329–332.
Article70. Rock EM, Sticht MA, Limebeer CL, Parker LA. Cannabinoid regulation of acute and anticipatory nausea. Cannabis Cannabinoid Res. 2016; 1:113–121.
Article71. Sticht MA, Limebeer CL, Rafla BR, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology. 2016; 102:92–102.
Article72. Sticht MA, Limebeer CL, Rafla BR, Parker LA. Intra-visceral insular cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide, suppresses acute nausea-induced conditioned gaping in rats. Neuroscience. 2015; 286:338–344.
Article73. Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999; 384:37–42.
Article74. Uranga JA, Vera G, Abalo R. Cannabinoid pharmacology and therapy in gut disorders. Biochem Pharmacol. 2018; 157:134–147.
Article75. Borrelli F, Romano B, Petrosino S, et al. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol. 2015; 172:142–158.
Article76. Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Application of carbon nanotubes as the carriers of the cannabinoid, 2-arachidonoylglycerol: towards a novel treatment strategy in colitis. Life Sci. 2017; 179:66–72.
Article77. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017; 389:1741–1755.
Article78. Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN. The incidence of fracture among patients with inflammatory bowel disease: a population-based cohort study. Ann Intern Med. 2000; 133:795–799.
Article79. Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005; 129:827–836.
Article80. Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009; 7:874–881.
Article81. Holzer P, Hassan AM, Jain P, Reichmann F, Farzi A. Neuroimmune pharmacological approaches. Curr Opin Pharmacol. 2015; 25:13–22.
Article82. Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011; 140:1827–1837.
Article83. Ioannidis O, Varnalidis I, Paraskevas G, Botsios D. Nutritional modulation of the inflammatory bowel response. Digestion. 2011; 84:89–101.
Article84. Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J Physiol Pharmacol. 2010; 61:89–97.85. Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009; 15:1678–1685.
Article86. Ke P, Shao BZ, Xu ZQ, et al. Activation of cannabinoid receptor 2 ameliorates DSS-induced colitis through inhibiting NLRP3 inflammasome in macrophages. PLoS One. 2016; 11:e0155076.
Article87. Storr MA, Keenan CM, Emmerdinger D, et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med (Berl). 2008; 86:925–936.
Article88. Shamran H, Singh NP, Zumbrun EE, et al. Fatty acid amide hydrolase (FAAH) blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav Immun. 2017; 59:10–20.
Article89. Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010; 126:21–38.
Article90. Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009; 87:1111–1121.
Article91. Tourteau A, Leleu-Chavain N, Body-Malapel M, et al. Switching cannabinoid response from CB(2) agonists to FAAH inhibitors. Bioorg Med Chem Lett. 2014; 24:1322–1326.
Article92. Sałaga M, Mokrowiecka A, Zakrzewski PK, et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J Crohns Colitis. 2014; 8:998–1009.
Article93. Sasso O, Migliore M, Habrant D, et al. Multitarget fatty acid amide hydrolase/cyclooxygenase blockade suppresses intestinal inflammation and protects against nonsteroidal anti-inflammatory drug-dependent gastrointestinal damage. FASEB J. 2015; 29:2616–2627.
Article94. Grill M, Hasenoehrl C, Kienzl M, Kargl J, Schicho R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem Cell Biol. 2019; 151:5–20.
Article95. Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011; 25:2711–2721.
Article96. Marquéz L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009; 4:e6893.
Article97. D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006; 20:568–570.
Article98. Harvey BS, Nicotra LL, Vu M, Smid SD. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine. 2013; 63:209–217.
Article99. Izzo AA, Capasso R, Aviello G, et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol. 2012; 166:1444–1460.
Article100. Grill M, Högenauer C, Blesl A, et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci Rep. 2019; 9:2358.
Article101. Suárez J, Romero-Zerbo Y, Márquez L, et al. Ulcerative colitis impairs the acylethanolamide-based anti-inflammatory system reversal by 5-aminosalicylic acid and glucocorticoids. PLoS One. 2012; 7:e37729.
Article102. Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G364–G371.
Article103. Storr M, Emmerdinger D, Diegelmann J, et al. The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn’s disease. PLoS One. 2010; 5:e9453.
Article104. Leinwand KL, Jones AA, Huang RH, et al. Cannabinoid receptor-2 ameliorates inflammation in murine model of Crohn’s disease. J Crohns Colitis. 2017; 11:1369–1380.
Article105. Stintzing S, Wissniowski TT, Lohwasser C, Alinger B, Neureiter D, Ocker M. Role of cannabinoid receptors and RAGE in inflammatory bowel disease. Histol Histopathol. 2011; 26:735–745.106. Romano B, Borrelli F, Fasolino I, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013; 169:213–229.
Article107. Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med. 2012; 18:615–625.
Article108. Capasso R, Borrelli F, Aviello G, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008; 154:1001–1008.
Article109. Lin XH, Yuece B, Li YY, et al. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil. 2011; 23:862–e342.
Article110. De Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011; 6:e28159.
Article111. Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012; 89:149–155.
Article112. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci. 2017; 62:1615–1620.
Article113. Pagano E, Capasso R, Piscitelli F, et al. An orally active Cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol. 2016; 7:341.
Article114. Irving PM, Iqbal T, Nwokolo C, et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis. 2018; 24:714–724.
Article115. Jamontt JM, Molleman A, Pertwee RG, Parsons ME. The effects of delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol. 2010; 160:712–723.
Article116. Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013; 11:1276–1280.
Article117. Izzo AA, Fezza F, Capasso R, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001; 134:563–570.
Article118. Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013; 85:1306–1316.
Article119. Casajuana Köguel C, López-Pelayo H, Balcells-Olivero MM, Colom J, Gual A. Psychoactive constituents of cannabis and their clinical implications: a systematic review. Adicciones. 2018; 30:140–151.120. Fichna J, Bawa M, Thakur GA, et al. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One. 2014; 9:e109115.
Article121. Lin S, Li Y, Shen L, et al. The anti-inflammatory effect and intestinal barrier protection of HU210 differentially depend on tlr4 signaling in dextran sulfate sodium-induced murine colitis. Dig Dis Sci. 2017; 62:372–386.
Article122. Li K, Fichna J, Schicho R, et al. A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology. 2013; 71:255–263.
Article123. Feng YJ, Li YY, Lin XH, Li K, Cao MH. Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J Gastroenterol. 2016; 22:9515–9524.
Article124. Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front Pharmacol. 2010; 1:132.
Article125. Bento AF, Marcon R, Dutra RC, et al. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol. 2011; 178:1153–1166.
Article126. El Bakali J, Muccioli GG, Body-Malapel M, et al. Conformational restriction leading to a selective CB2 cannabinoid receptor agonist orally active against colitis. ACS Med Chem Lett. 2014; 6:198–203.
Article127. Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol. 2012; 258:256–267.
Article128. Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015; 172:4790–4805.
Article129. Rhee MH, Vogel Z, Barg J, et al. Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem. 1997; 40:3228–3233.
Article130. Gugliandolo A, Pollastro F, Grassi G, Bramanti P, Mazzon E. In vitro model of neuroinflammation: efficacy of cannabigerol, a non-psychoactive cannabinoid. Int J Mol Sci. 2018; 19:1992.
Article131. Amin MR, Ali DW. Pharmacology of medical cannabis. Adv Exp Med Biol. 2019; 1162:151–165.
Article132. Andrzejak V, Muccioli GG, Body-Malapel M, et al. New FAAH inhibitors based on 3-carboxamido-5-aryl-isoxazole scaffold that protect against experimental colitis. Bioorg Med Chem. 2011; 19:3777–3786.
Article133. Fichna J, Sałaga M, Stuart J, et al. Selective inhibition of FAAH produces antidiarrheal and antinociceptive effect mediated by endocannabinoids and cannabinoid-like fatty acid amides. Neurogastroenterol Motil. 2014; 26:470–481.
Article134. Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006; 58:389–462.
Article135. Casu MA, Porcella A, Ruiu S, et al. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003; 459:97–105.
Article136. Duncan M, Mouihate A, Mackie K, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G78–G87.137. Lee Y, Jo J, Chung HY, Pothoulakis C, Im E. Endocannabinoids in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G655–G666.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenesis of Inflammatory Bowel Diseases
- Mechanism-based Drug Therapy of Inflammatory Bowel Disease With Special Reference to Rheumatic Disease
- Inflammatory Bowel Disease in Asia: The Challenges and Opportunities
- The analgesic and anti-inflammatory effects of a combined preparation based on the blunt-nosed viper’s venom and oregano essential oil
- Advances in Management of Intestinal Behçet’s Disease: A Perspective From Gastroenterologists