J Yeungnam Med Sci.
2022 Oct;39(4):300-308. 10.12701/jyms.2021.01648.
Clinical impact of spine magnetic resonance imaging as a valuable prognostic tool for patients with multiple myeloma: a retrospective study
- Affiliations
-
- 1Department of Hematology-Oncology, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Department of Radiology, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2534657
- DOI: http://doi.org/10.12701/jyms.2021.01648
Abstract
- Background
This study investigated the prognostic impact of spine magnetic resonance imaging (MRI) in patients newly diagnosed with multiple myeloma (MM).
Methods
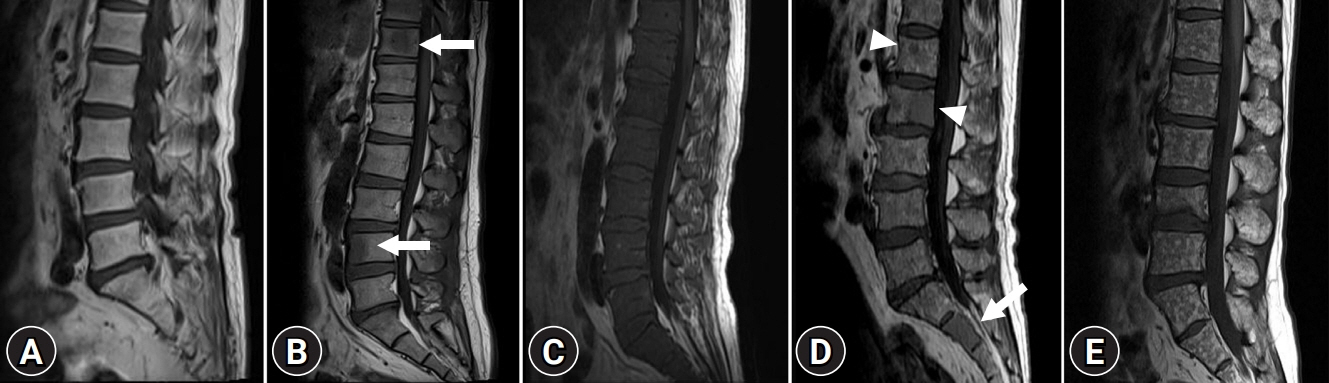
We retrospectively evaluated 214 patients who were newly diagnosed with MM between March 2015 and December 2019. The patients were classified into five different infiltration patterns based on spine MRI as follows: (1) normal appearance, (2) focal, (3) diffuse, (4) combined focal and diffuse infiltration, and (5) “salt-and-pepper.”
Results
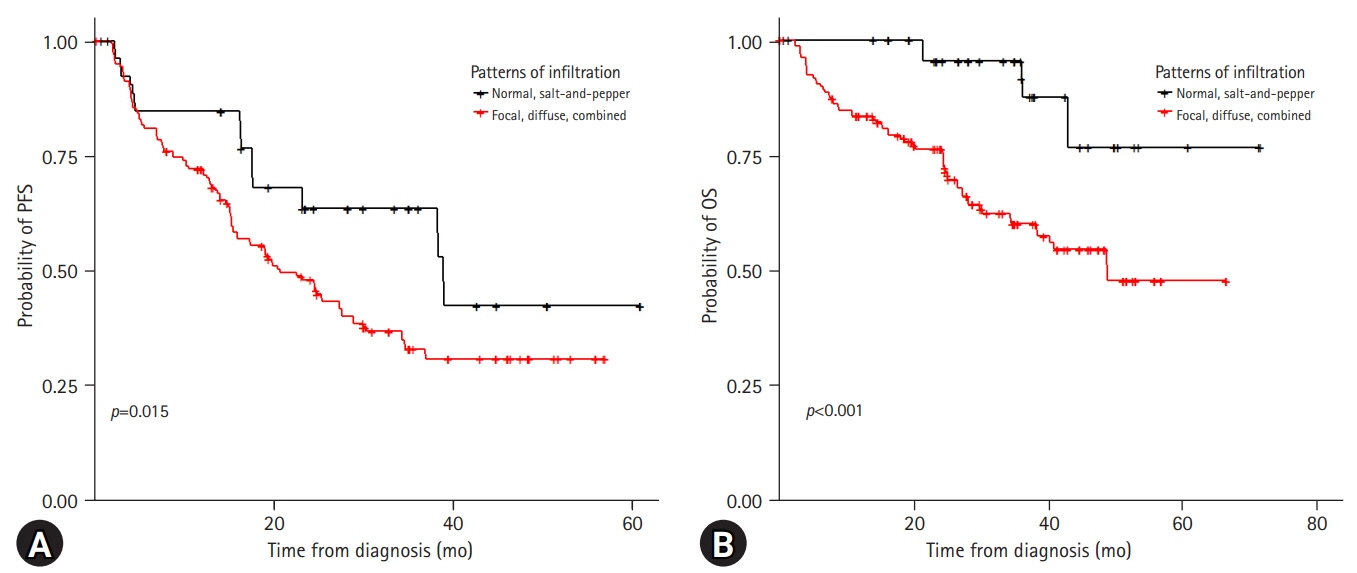
Forty patients (18.7%) showed a normal appearance, whereas focal, diffuse, combined focal and diffuse infiltration, and “salt-and-pepper” patterns were identified in 68 (31.8%), 40 (18.7%), 52 (24.3%), and 14 patients (6.5%), respectively. The patients with normal and “salt-and-pepper” patterns were younger than patients with other patterns (median age, 61.6 vs. 66.8 years; p=0.001). Moreover, 63% and 59.3% of patients with normal and “salt-and-pepper” patterns were scored International Staging System (ISS) stage I and revised ISS (R-ISS) stage I, respectively, whereas only 12.5% of patients with other patterns were scored ISS stage I and R-ISS stage I. Patients with normal and “salt-and-pepper” patterns had a better prognosis than those with other patterns, whereas relapse and death rates were significantly higher in patients with focal, diffuse, and combined MRI patterns.
Conclusion
Characteristic MRI findings have a significant prognostic value for long-term survival in patients newly diagnosed with MM. In particular, focal, diffuse, and combined focal and diffuse infiltration patterns are unfavorable prognostic factors.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.
Article2. Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010; 376:2075–85.
Article3. Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010; 116:679–86.
Article4. Cardona-Benavides IJ, de Ramón C, Gutiérrez NC. Genetic abnormalities in multiple myeloma: prognostic and therapeutic implications. Cells. 2021; 10:336.
Article5. Usmani SZ, Hoering A, Cavo M, Miguel JS, Goldschimdt H, Hajek R, et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma: an IMWG Research Project. Blood Cancer J. 2018; 8:123.6. Dimopoulos MA, Kastritis E, Michalis E, Tsatalas C, Michael M, Pouli A, et al. The International Scoring System (ISS) for multiple myeloma remains a robust prognostic tool independently of patients’ renal function. Ann Oncol. 2012; 23:722–9.
Article7. Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015; 33:2863–9.
Article8. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15:e538–48.
Article9. Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007; 25:1121–8.
Article10. Bäuerle T, Hillengass J, Fechtner K, Zechmann CM, Grenacher L, Moehler TM, et al. Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging. Radiology. 2009; 252:477–85.
Article11. Ailawadhi S, Abdelhalim AN, Derby L, Mashtare TL, Miller KC, Wilding GE, et al. Extent of disease burden determined with magnetic resonance imaging of the bone marrow is predictive of survival outcome in patients with multiple myeloma. Cancer. 2010; 116:84–92.
Article12. Moulopoulos LA, Dimopoulos MA, Christoulas D, Kastritis E, Anagnostou D, Koureas A, et al. Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia. 2010; 24:1206–12.
Article13. Moulopoulos LA, Gika D, Anagnostopoulos A, Delasalle K, Weber D, Alexanian R, et al. Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol. 2005; 16:1824–8.
Article14. Song IC, Kim JN, Choi YS, Ryu H, Lee MW, Lee HJ, et al. Diagnostic and prognostic implications of spine magnetic resonance imaging at diagnosis in patients with multiple myeloma. Cancer Res Treat. 2015; 47:465–72.
Article15. Dutoit JC, Verstraete KL. MRI in multiple myeloma: a pictorial review of diagnostic and post-treatment findings. Insights Imaging. 2016; 7:553–69.
Article16. Narquin S, Ingrand P, Azais I, Delwail V, Vialle R, Boucebci S, et al. Comparison of whole-body diffusion MRI and conventional radiological assessment in the staging of myeloma. Diagn Interv Imaging. 2013; 94:629–36.
Article17. Terpos E, Morgan G, Dimopoulos MA, Drake MT, Lentzsch S, Raje N, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013; 31:2347–57.
Article18. Xiao W, Wang Y, Pacios S, Li S, Graves DT. Cellular and molecular aspects of bone remodeling. Front Oral Biol. 2016; 18:9–16.
Article19. Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011; 17:1231–4.
Article20. Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016; 76:1089–100.
Article21. Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010; 30:127–42.
Article22. Kusumoto S, Jinnai I, Itoh K, Kawai N, Sakata T, Matsuda A, et al. Magnetic resonance imaging patterns in patients with multiple myeloma. Br J Haematol. 1997; 99:649–55.23. Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple myeloma. Leukemia. 2009; 23:1545–56.
Article24. Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007; 92:50–5.
Article25. Delorme S, Baur-Melnyk A. Imaging in multiple myeloma. Recent Results Cancer Res. 2011; 183:133–47.
Article26. Baur-Melnyk A, Buhmann S, Becker C, Schoenberg SO, Lang N, Bartl R, et al. Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am J Roentgenol. 2008; 190:1097–104.
Article27. Moulopoulos LA, Dimopoulos MA, Kastritis E, Christoulas D, Gkotzamanidou M, Roussou M, et al. Diffuse pattern of bone marrow involvement on magnetic resonance imaging is associated with high risk cytogenetics and poor outcome in newly diagnosed, symptomatic patients with multiple myeloma: a single center experience on 228 patients. Am J Hematol. 2012; 87:861–4.
Article28. Hillengass J, Zechmann CM, Nadler A, Hose D, Cremer FW, Jauch A, et al. Gain of 1q21 and distinct adverse cytogenetic abnormalities correlate with increased microcirculation in multiple myeloma. Int J Cancer. 2008; 122:2871–5.
Article29. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996; 335:91–7.
Article30. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003; 348:1875–83.
Article31. Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007; 13:183–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion-Weighted Magnetic Resonance Imaging of Spine
- Analysis of Bone Mineral Density in Multiple Myeloma: A Comparison of Bone Mineral Density with Plain Radiography, Magnetic Resonance Imaging, and Clinical Staging
- A Case of Nonsecretory Multiple Myeloma with Atypical Imaging Features
- Diagnostic and Prognostic Implications of Spine Magnetic Resonance Imaging at Diagnosis in Patients with Multiple Myeloma
- Nonsecretory Multiple Myeloma with Multiple Spine Fracture