J Korean Med Sci.
2022 Sep;37(37):e276. 10.3346/jkms.2022.37.e276.
Role of Autophagy in the Pathogenesis of Diabetes and Therapeutic Potential of Autophagy Modulators in the Treatment of Diabetes and Metabolic Syndrome
- Affiliations
-
- 1Soonchunhyang Institute of Medi-bio Science and Division of Endocrinology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea
- KMID: 2533555
- DOI: http://doi.org/10.3346/jkms.2022.37.e276
Abstract
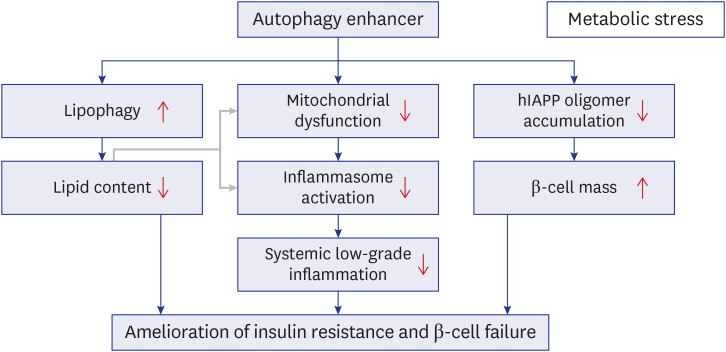
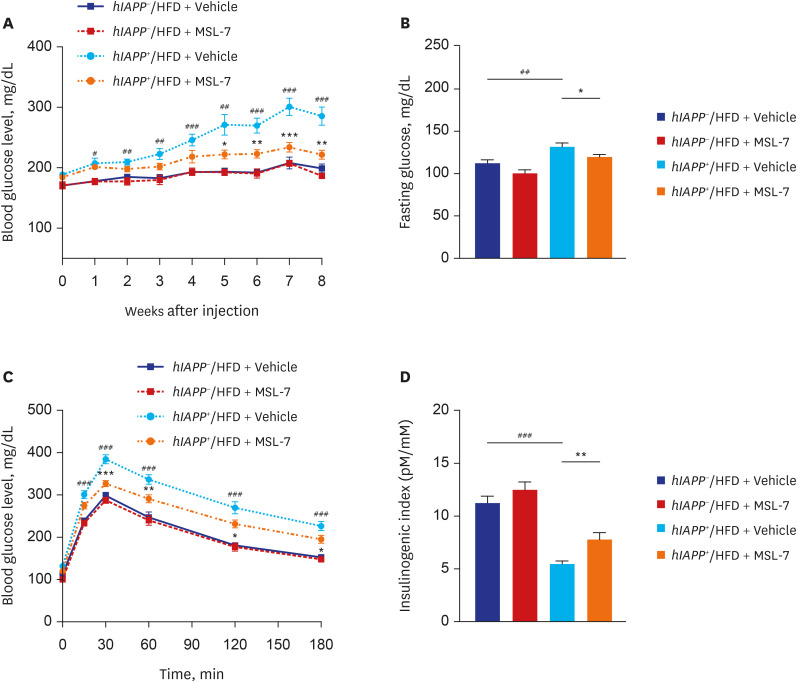
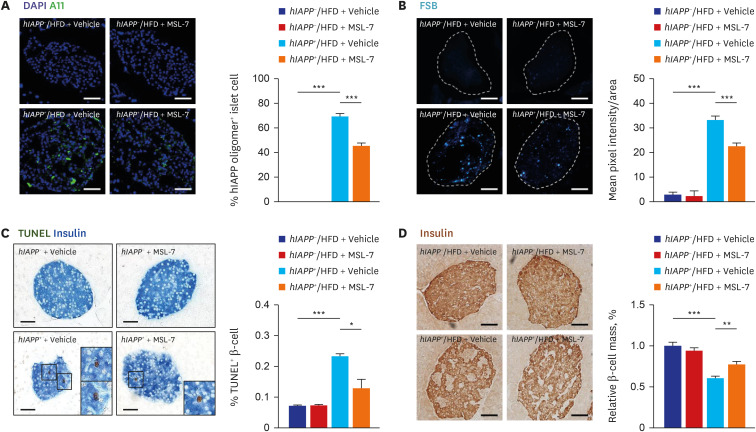
- Autophagy is critically involved in the maintenance of intracellular nutrient homeostasis and organelle function. Dysregulated autophagy is likely to play a role in the development of metabolic disorders and diabetes because autophagy is critical in the rejuvenation of dysfunctional or stressed endoplasmic reticulum and mitochondria that play a crucial role in the development of diabetes. Indeed, systemic autophagy insufficiency led to the increased tissue lipid content, aggravated metabolic and finally more severe diabetes when metabolic stress was imposed, suggesting that autophagy insufficiency of dysfunction of lysosome, an effector organelle of autophagy, due to aging, genetic predisposition or environmental factors could be an underlying cause of diabetes. Conversely, autophagy enhancer could improve metabolic profile of obese mice by reducing tissue lipid content and ameliorating metabolic inflammation. Furthermore, clearance of human islet amyloid polypeptide (hIAPP) oligomer and amyloid that accumulate in pancreatic islets of > 90% of diabetes patients was also dependent on autophagy. Consistently, autophagy enhancer could improve glucose profile and β-cell function of transgenic mice expressing amyloidogenic hIAPP in pancreatic β-cells, which was accompanied by reduced accumulation of hIAPP oligomer or amyloid, ameliorated β-cell apoptosis and increased β-cell mass. These results suggest that autophagy enhancer could be a novel therapeutic modality against diabetes associated with lipid overload and human diabetes characterized by islet amyloid accumulation.
Keyword
Figure
Reference
-
1. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000; 290(5497):1717–1721. PMID: 11099404.
Article2. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147(4):728–741. PMID: 22078875.
Article3. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006; 441(7095):885–889. PMID: 16625204.
Article4. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006; 441(7095):880–884. PMID: 16625205.
Article5. Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962; 12(1):198–202. PMID: 13862833.
Article6. Deter RL, De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967; 33(2):437–449. PMID: 4292315.
Article7. Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol. 1978; 78(1):152–167. PMID: 670291.
Article8. Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993; 333(1-2):169–174. PMID: 8224160.
Article9. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021; 17(1):1–382. PMID: 33634751.10. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019; 176(1-2):11–42. PMID: 30633901.
Article11. Park K, Lee MS. Current status of autophagy enhancers in metabolic disorders and other diseases. Front Cell Dev Biol. 2022; 10:811701. PMID: 35237600.
Article12. Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012; 11(9):709–730. PMID: 22935804.
Article13. Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013; 494(7436):201–206. PMID: 23364696.
Article14. Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010; 465(7300):942–946. PMID: 20526321.
Article15. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009; 9(1):35–51. PMID: 19117545.
Article16. Özcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306(5695):457–461. PMID: 15486293.
Article17. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009; 361(22):2143–2152. PMID: 19940299.
Article18. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003; 300(5622):1140–1142. PMID: 12750520.
Article19. Gubas A, Dikic I. A guide to the regulation of selective autophagy receptors. FEBS J. 2022; 289(1):75–89. PMID: 33730405.
Article20. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008; 183(5):795–803. PMID: 19029340.
Article21. Kim J, Lim YM, Lee MS. The role of autophagy in systemic metabolism and human-type diabetes. Mol Cells. 2018; 41(1):11–17. PMID: 29370692.22. Yasukochi Y, Sakuma J, Takeuchi I, Kato K, Oguri M, Fujimaki T, et al. Two novel susceptibility loci for type 2 diabetes mellitus identified by longitudinal exome-wide association studies in a Japanese population. Genomics. 2019; 111(1):34–42. PMID: 29273463.
Article23. Kim KH, Lee MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014; 10(6):322–337. PMID: 24663220.
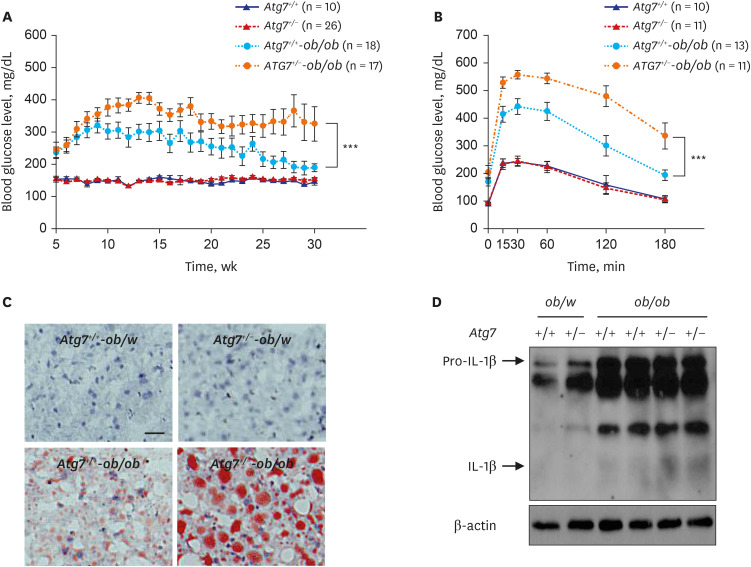
Article24. Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun. 2014; 5(1):4934. PMID: 25255859.
Article25. Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013; 14(5):454–460. PMID: 23502856.
Article26. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011; 17(2):179–188. PMID: 21217695.
Article27. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011; 12(5):408–415. PMID: 21478880.
Article28. Kim J, Kim SH, Kang H, Lee S, Park SY, Cho Y, et al. TFEB-GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat Metab. 2021; 3(3):410–427. PMID: 33758420.
Article29. Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008; 45(3):231–241. PMID: 18482593.
Article30. Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011; 473(7348):528–531. PMID: 21532591.
Article31. Fernández AF, Bárcena C, Martínez-García GG, Tamargo-Gómez I, Suárez MF, Pietrocola F, et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017; 8(8):e2970. PMID: 28771229.
Article32. He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012; 481(7382):511–515. PMID: 22258505.
Article33. Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013; 4(1):2300. PMID: 23939249.
Article34. Castillo K, Nassif M, Valenzuela V, Rojas F, Matus S, Mercado G, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013; 9(9):1308–1320. PMID: 23851366.
Article35. Dehay B, Bové J, Rodríguez-Muela N, Perier C, Recasens A, Boya P, et al. Pathogenic lysosomal depletion in Parkin’s disease. J Neurosci. 2010; 30(37):12534–12544.36. Lin F, Ghislat G, Luo S, Renna M, Siddiqi F, Rubinsztein DC. XIAP and cIAP1 amplifications induce Beclin 1-dependent autophagy through NFκB activation. Hum Mol Genet. 2015; 24(10):2899–2913. PMID: 25669656.
Article37. Xie Q, Lin Q, Li D, Chen J. Imatinib induces autophagy via upregulating XIAP in GIST882 cells. Biochem Biophys Res Commun. 2017; 488(4):584–589. PMID: 28528977.
Article38. Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JA, Lovat PE, Arden C. Glucagon-Like Peptide 1 protects pancreatic β-cells from death by increasing autophagic flux and restoring lysosomal function. Diabetes. 2017; 66(5):1272–1285. PMID: 28232493.
Article39. Zummo FP, Krishnanda SI, Georgiou M, O’Harte FP, Parthsarathy V, Cullen KS, et al. Exendin-4 stimulates autophagy in pancreatic β-cells via the RAPGEF/EPAC-Ca 2+-PPP3/calcineurin-TFEB axis. Autophagy. Forthcoming. 2021.40. Farkas T, Høyer-Hansen M, Jäättelä M. Identification of novel autophagy regulators by a luciferase-based assay for the kinetics of autophagic flux. Autophagy. 2009; 5(7):1018–1025. PMID: 19652534.
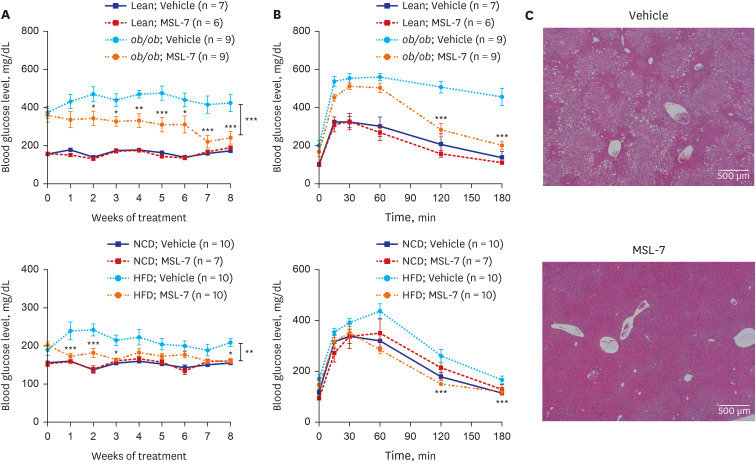
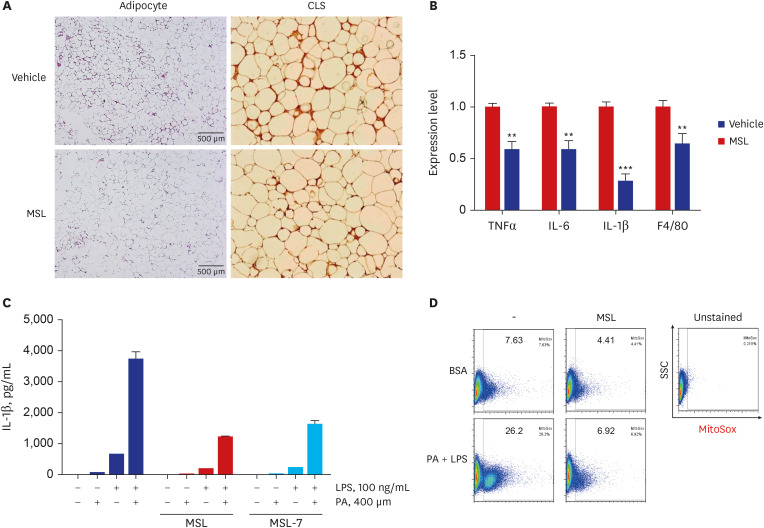
Article41. Lim H, Lim YM, Kim KH, Jeon YE, Park K, Kim J, et al. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nat Commun. 2018; 9(1):1438. PMID: 29650965.
Article42. Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013; 62(8):2674–2682. PMID: 23881200.
Article43. Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006; 22(2):159–168. PMID: 16603397.
Article44. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011; 332(6036):1429–1433. PMID: 21617040.45. Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999; 48(2):241–253. PMID: 10334297.
Article46. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011; 91(3):795–826. PMID: 21742788.
Article47. Gebre-Medhin S, Olofsson C, Mulder H. Islet amyloid polypeptide in the islets of Langerhans: friend or foe? Diabetologia. 2000; 43(6):687–695. PMID: 10907112.
Article48. Venkatanarayan A, Raulji P, Norton W, Chakravarti D, Coarfa C, Su X, et al. IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo. Nature. 2015; 517(7536):626–630. PMID: 25409149.
Article49. Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006; 443(7113):780–786. PMID: 17051204.
Article50. Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996; 93(14):7283–7288. PMID: 8692984.
Article51. Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996; 93(8):3492–3496. PMID: 8622964.
Article52. Kim J, Cheon H, Jeong YT, Quan W, Kim KH, Cho JM, et al. Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes. J Clin Invest. 2014; 124(8):3311–3324. PMID: 25036705.
Article53. Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014; 124(8):3489–3500. PMID: 25036708.
Article54. Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, et al. Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy. J Clin Invest. 2014; 124(8):3634–3644. PMID: 25036706.
Article55. Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008; 29(3):303–316. PMID: 18314421.
Article56. Paulsson JF, Andersson A, Westermark P, Westermark GT. Intracellular amyloid-like deposits contain unprocessed pro-islet amyloid polypeptide (proIAPP) in beta cells of transgenic mice overexpressing the gene for human IAPP and transplanted human islets. Diabetologia. 2006; 49(6):1237–1246. PMID: 16570161.
Article57. Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005; 44(36):12113–12119. PMID: 16142909.
Article58. Park K, Verchere CB. Identification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide. J Biol Chem. 2001; 276(20):16611–16616. PMID: 11145957.
Article59. Kim J, Park K, Kim MJ, Lim H, Kim KH, Kim SW, et al. An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer. Nat Commun. 2021; 12(1):183. PMID: 33420039.
Article60. Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007; 2(1):18–28. PMID: 17897471.61. Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003; 300(5618):486–489. PMID: 12702875.
Article62. Cunningham CN, Williams JM, Knupp J, Arunagiri A, Arvan P, Tsai B. Cells deploy a two-pronged strategy to rectify misfolded proinsulin aggregates. Mol Cell. 2019; 75(3):442–456.e4. PMID: 31176671.
Article63. Arunagiri A, Haataja L, Pottekat A, Pamenan F, Kim S, Zeltser LM, et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife. 2019; 8:e44532. PMID: 31184302.
Article64. Bachar-Wikstrom E, Wikstrom JD, Ariav Y, Tirosh B, Kaiser N, Cerasi E, et al. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013; 62(4):1227–1237. PMID: 23274896.
Article65. Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005; 280(49):40406–40416. PMID: 16221682.
Article66. Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012; 335(6076):1638–1643. PMID: 22461615.
Article67. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005; 307(5712):1098–1101. PMID: 15718470.
Article68. Park K, Lim H, Kim J, Hwang H, Lee YS, Bae SH, et al. Essential role of lysosomal Ca2+-mediated TFEB activation in mitophagy and functional adaptation of pancreatic b-cells to metabolic stress. Nat Commun. 2022; 13:1300. PMID: 35288580.69. Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, et al. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell. 2014; 157(7):1577–1590. PMID: 24949970.
Article70. Ko MS, Yun JY, Baek IJ, Jang JE, Hwang JJ, Lee SE, et al. Mitophagy deficiency increases NLRP3 to induce brown fat dysfunction in mice. Autophagy. 2021; 17(5):1205–1221. PMID: 32400277.
Article71. Wu H, Wang Y, Li W, Chen H, Du L, Liu D, et al. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy. 2019; 15(11):1882–1898. PMID: 30898010.
Article72. Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy. 2016; 12(12):2467–2483. PMID: 27813694.
Article73. Fernando R, Castro JP, Flore T, Deubel S, Grune T, Ott C. Age-related maintenance of the autophagy-lysosomal system Is dependent on skeletal muscle type. Oxid Med Cell Longev. 2020; 2020:4908162. PMID: 32774673.
Article74. Hütter E, Skovbro M, Lener B, Prats C, Rabøl R, Dela F, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007; 6(2):245–256. PMID: 17376148.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Autophagy in the Control of Body Metabolism
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Nutritional Status and Cardiac Autophagy
- Interplay Between Primary Cilia and Autophagy and Its Controversial Roles in Cancer
- Endothelial cell autophagy in the context of disease development