J Korean Neurosurg Soc.
2022 Sep;65(5):665-679. 10.3340/jkns.2021.0101.
Phosphodiesterase-5 Inhibitor Attenuates Anxious Phenotypes and Movement Disorder Induced by Mild Ischemic Stroke in Rats
- Affiliations
-
- 1Department of Anatomy, College of Medicine, Soonchunhyang University, Cheonan, Korea
- 2BK21 FOUR Project, College of Medicine, Soonchunhyang University, Cheonan, Korea
- 3Graduate School of New Drug Discovery & Development, Chungnam National University, Daejeon, Korea
- 4Soonchunhyang Institute of Medi-bio Science (SIMS), Soonchunhyang University, Cheonan, Korea
- 5Department of Neurosurgery, Cheonan Hospital, College of Medicine, Soonchunhyang University, Cheonan, Korea
- KMID: 2533029
- DOI: http://doi.org/10.3340/jkns.2021.0101
Abstract
Objective
: Patients with mild ischemic stroke experience various sequela and residual symptoms, such as anxious behavior and deficits in movement. Few approaches have been proved to be effective and safe therapeutic approaches for patients with mild ischemic stroke by acute stroke. Sildenafil (SIL), a phosphodiesterase-5 inhibitor (PDE5i), is a known remedy for neurodegenerative disorders and vascular dementia through its angiogenesis and neurogenesis effects. In this study, we investigated the efficacy of PDE5i in the emotional and behavioral abnormalities in rats with mild ischemic stroke.
Methods
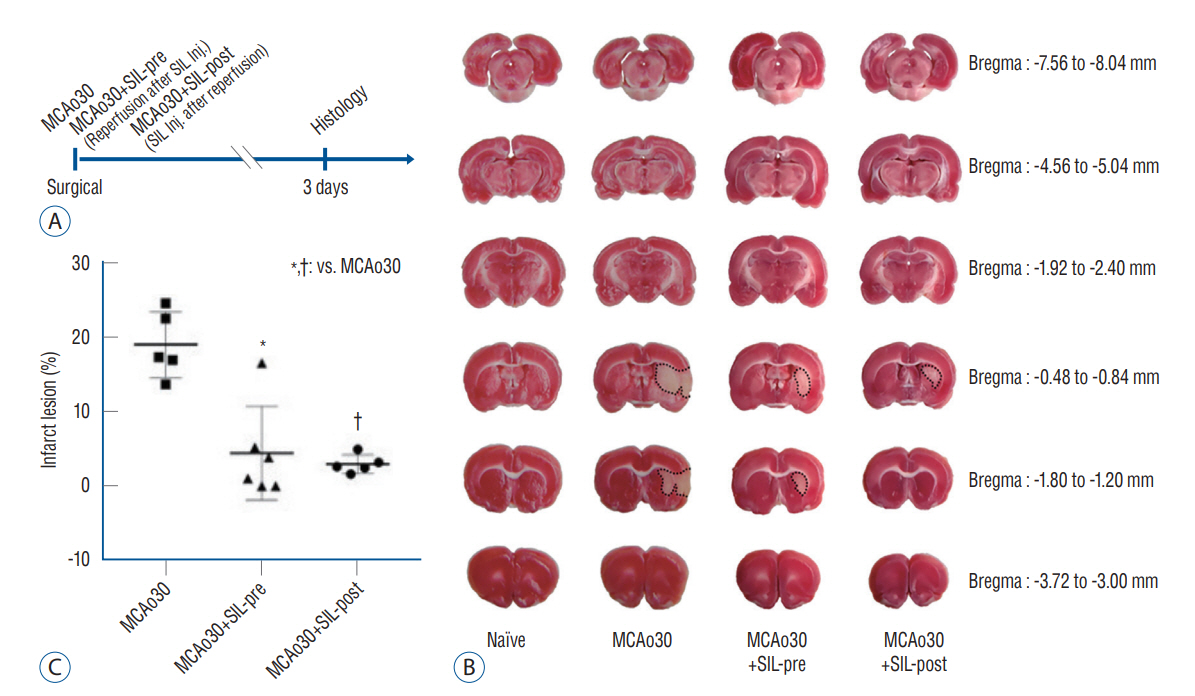
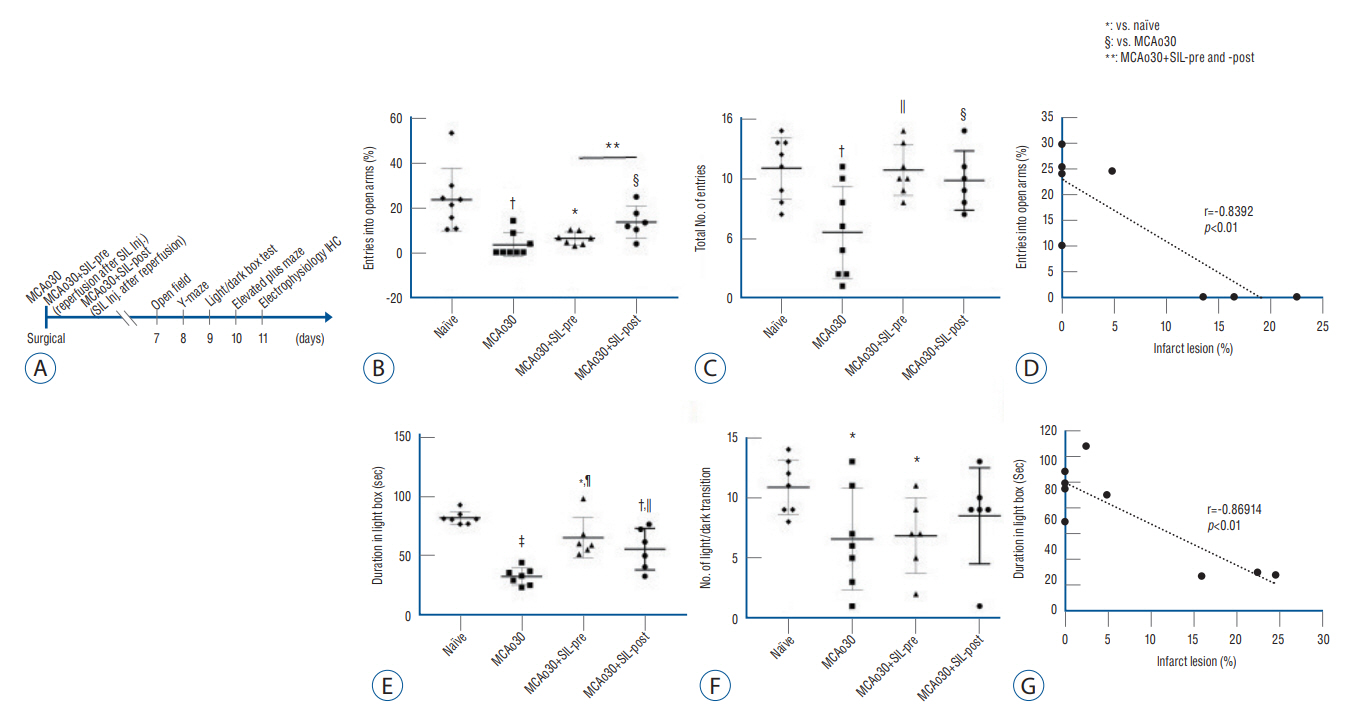
: We divided the rats into four groups as follows (n=20, respectively) : group 1, naïve; group 2, middle cerebral artery occlusion (MCAo30); group 3, MCAo30+SIL-pre; and group 4, MCAo30+SIL-post. In the case of drug administration groups, single dose of PDE5i (sildenafil citrate, 20 mg/kg) was given at 30-minute before and after reperfusion of MCAo in rats. After surgery, we investigated and confirmed the therapeutic effect of sildenafil on histology, immunofluorescence, behavioral assays and neural oscillations.
Results
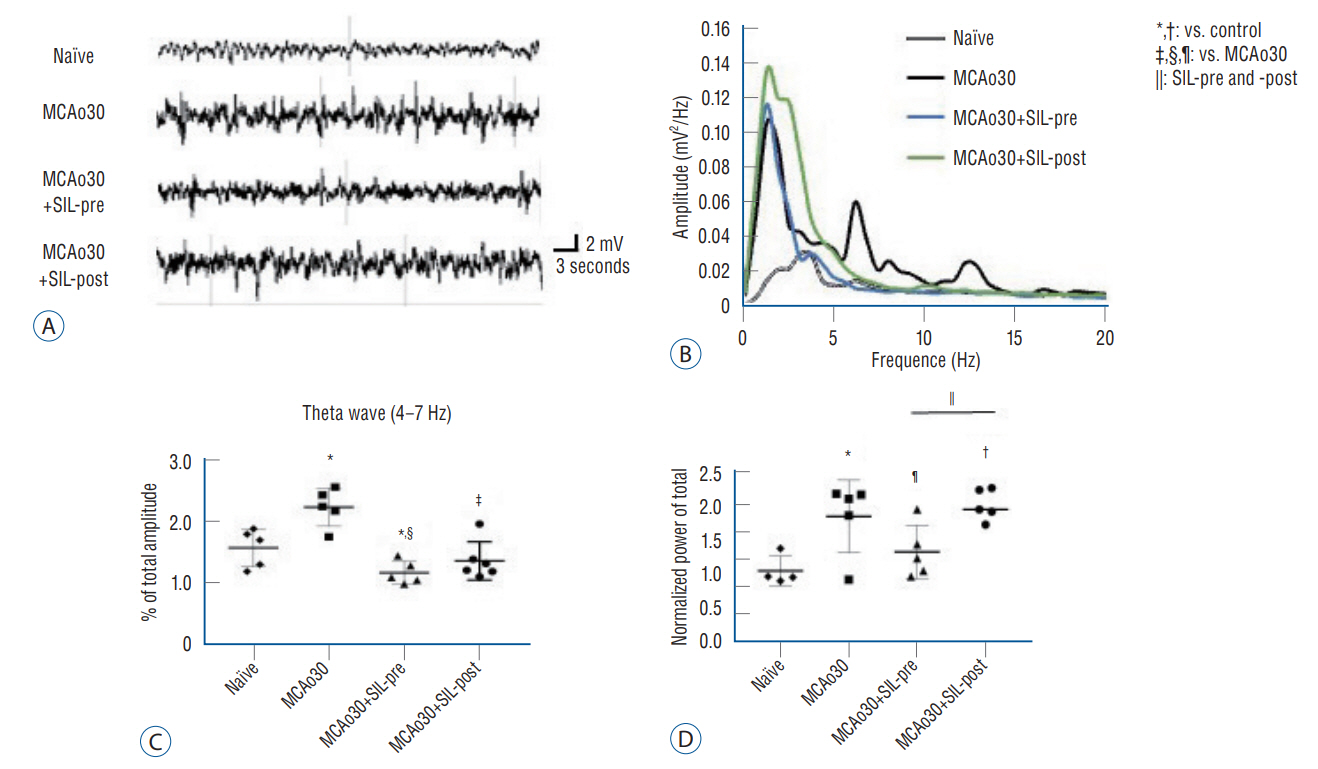
: Sildenafil alleviated a neuronal loss and reduced the infarction volume. And results of behavior task and immunofluorescence shown possibility that anti-inflammation process and improve motor deficits sildenafil treatment after mild ischemic stroke. Furthermore, sildenafil treatment attenuated the alteration of theta-frequency rhythm in the CA1 region of the hippocampus, a known neural oscillatory marker for anxiety disorder in rodents, induced by mild ischemic stroke.
Conclusion
: PDE5i as effective therapeutic agents for anxiety and movement disorders and provide robust preclinical evidence to support the development and use of PDE5i for the treatment of mild ischemic stroke residual disorders.
Keyword
Figure
Reference
-
References
1. Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 65:257–269. 2010.2. Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron. 71:898–910. 2011.3. Ala M, Mohammad Jafari R, Dehpour AR. Sildenafil beyond erectile dysfunction and pulmonary arterial hypertension: thinking about new indications. Fundam Clin Pharmacol. 35:235–259. 2021.4. Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 100:2106–2111. 2003.5. Ascah A, Khairallah M, Daussin F, Bourcier-Lucas C, Godin R, Allen BG, et al. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am J Physiol Heart Circ Physiol. 300:H144–H153. 2011.6. Bak J, Pyeon HI, Seok JI, Choi YS. Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation. Behav Brain Res. 320:219–224. 2017.7. Barger SW, Van Eldik LJ. S100 beta stimulates calcium fluxes in glial and neuronal cells. J Biol Chem. 267:9689–9694. 1992.8. Baudier J, Delphin C, Grunwald D, Khochbin S, Lawrence JJ. Characterization of the tumor suppressor protein p53 as a protein kinase C substrate and a S100b-binding protein. Proc Natl Acad Sci U S A. 89:11627–11631. 1992.9. Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 17:1304–1308. 1986.10. Bogousslavsky J, Martin R, Regli F, Despland PA, Bolyn S. Persistent worsening of stroke sequelae after delayed seizures. Arch Neurol. 49:385–388. 1992.11. Borah M, Dhar S, Gogoi DM, Ruram AA. Association of serum calcium levels with infarct size in acute ischemic stroke: observations from northeast india. J Neurosci Rural Pract. 7(Suppl 1):S41–S45. 2016.12. Buck BH, Liebeskind DS, Saver JL, Bang OY, Starkman S, Ali LK, et al. Association of higher serum calcium levels with smaller infarct volumes in acute ischemic stroke. Arch Neurol. 64:1287–1291. 2007.13. Charriaut-Marlangue C, Nguyen T, Bonnin P, Duy AP, Leger PL, Csaba Z, et al. Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia. Stroke. 45:850–856. 2014.14. Chen XM, Wang NN, Zhang TY, Wang F, Wu CF, Yang JY. Neuroprotection by sildenafil: neuronal networks potentiation in acute experimental stroke. CNS Neurosci Ther. 20:40–49. 2014.15. Chung JW, Ryu WS, Kim BJ, Yoon BW. Elevated calcium after acute ischemic stroke: association with a poor short-term outcome and longterm mortality. J Stroke. 17:54–59. 2015.16. Ciani E, Guidi S, Bartesaghi R, Contestabile A. Nitric oxide regulates cGMP-dependent cAMP-responsive element binding protein phosphorylation and Bcl-2 expression in cerebellar neurons: implication for a survival role of nitric oxide. J Neurochem. 82:1282–1289. 2002.17. Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 274:13729–13732. 1999.18. Cruz-Burgos M, Losada-Garcia A, Cruz-Hernández CD, Cortés-Ramírez SA, Camacho-Arroyo I, Gonzalez-Covarrubias V, et al. New approaches in oncology for repositioning drugs: the case of PDE5 inhibitor sildenafil. Front Oncol. 11:627229. 2021.19. Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 17:197–218. 2010.20. Eroğlu L, Cağlayan B. Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol Res. 36:381–385. 1997.21. Friedman B, Schachtrup C, Tsai PS, Shih AY, Akassoglou K, Kleinfeld D, et al. Acute vascular disruption and aquaporin 4 loss after stroke. Stroke. 40:2182–2190. 2009.22. Frydenlund DS, Bhardwaj A, Otsuka T, Mylonakou MN, Yasumura T, Davidson KG, et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci U S A. 103:13532–13536. 2006.23. Gangadharan G, Shin J, Kim SW, Kim A, Paydar A, Kim DS, et al. Medial septal GABAergic projection neurons promote object exploration behavior and type 2 theta rhythm. Proc Natl Acad Sci U S A. 113:6550–6555. 2016.24. García-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA, et al. Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 64:114–123. 2013.25. Gilhotra N, Dhingra D. Involvement of NO-cGMP pathway in antianxiety effect of aminoguanidine in stressed mice. Prog Neuropsychopharmacol Biol Psychiatry. 33:1502–1507. 2009.26. Gill R, Sibson NR, Hatfield RH, Burdett NG, Carpenter TA, Hall LD, et al. A comparison of the early development of ischaemic damage following permanent middle cerebral artery occlusion in rats as assessed using magnetic resonance imaging and histology. J Cereb Blood Flow Metab. 15:1–11. 1995.27. Gómez-Vallejo V, Ugarte A, García-Barroso C, Cuadrado-Tejedor M, Szczupak B, Dopeso-Reyes IG, et al. Pharmacokinetic investigation of sildenafil using positron emission tomography and determination of its effect on cerebrospinal fluid cGMP levels. J Neurochem. 136:403–415. 2016.28. Gordon JA, Lacefield CO, Kentros CG, Hen R. State-dependent alterations in hippocampal oscillations in serotonin 1A receptor-deficient mice. J Neurosci. 25:6509–6519. 2005.29. Hoeller AA, Duzzioni M, Duarte FS, Leme LR, Costa AP, Santos EC, et al. GABA-A receptor modulators alter emotionality and hippocampal theta rhythm in an animal model of long-lasting anxiety. Brain Res. 1532:21–31. 2013.30. Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol. 47:157–160. 1990.31. Kim KK, Kim DG, Ku YH, Lee YJ, Kim WC, Kim OJ, et al. Bilateral cerebral hemispheric infarction associated with sildenafil citrate (Viagra) use. Eur J Neurol. 15:306–308. 2008.32. Koh HY, Kim D, Lee J, Lee S, Shin HS. Deficits in social behavior and sensorimotor gating in mice lacking phospholipase Cbeta1. Genes Brain Behav. 7:120–128. 2008.33. Kolettis TM, Kontaras K, Spartinos I, Maniotis C, Varnavas V, Koutouzis M, et al. Dose-dependent effects of sildenafil on post-ischaemic left ventricular function in the rat isolated heart. J Pharm Pharmacol. 62:346–351. 2010.34. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet Glob Health. 1:e259–e281. 2013.35. Kruuse C, Khurana TS, Rybalkin SD, Birk S, Engel U, Edvinsson L, et al. Phosphodiesterase 5 and effects of sildenafil on cerebral arteries of man and guinea pig. Eur J Pharmacol. 521:105–114. 2005.36. Kwak SE, Kim JE, Kim SC, Kwon OS, Choi SY, Kang TC. Hyperthermic seizure induces persistent alteration in excitability of the dentate gyrus in immature rats. Brain Res. 1216:1–15. 2008.37. Liebenberg N, Harvey BH, Brand L, Wegener G, Brink CB. Chronic treatment with the phosphodiesterase type 5 inhibitors sildenafil and tadalafil display anxiolytic effects in flinders sensitive line rats. Metab Brain Dis. 27:337–340. 2012.38. Lu H, Hu H, He ZP. Reperfusion of the rat brain tissues following acute ischemia: the correlation among diffusion-weighted imaging, histopathology, and aquaporin-4 expression. Chin Med J (Engl). 124:3148–3153. 2011.39. Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to latephase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 19:10250–10261. 1999.40. Ludhiadch A, Sharma R, Muriki A, Munshi A. Role of calcium homeostasis in ischemic stroke: a review. CNS Neurol Disord Drug Targets. 21:52–61. 2022.41. Moran GM, Fletcher B, Feltham MG, Calvert M, Sackley C, Marshall T. Fatigue, psychological and cognitive impairment following transient ischaemic attack and minor stroke: a systematic review. Eur J Neurol. 21:1258–1267. 2014.42. Moretti R, Leger PL, Besson VC, Csaba Z, Pansiot J, Di Criscio L, et al. Sildenafil, a cyclic GMP phosphodiesterase inhibitor, induces microglial modulation after focal ischemia in the neonatal mouse brain. J Neuroinflammation. 13:95. 2016.43. Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J Cell Biol. 160:719–727. 2003.44. Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 93:1543–1562. 2013.45. Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, et al. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res. 71:485–495. 2003.46. Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 17:171–180. 1997.47. O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 59:467–477. 2006.48. Ovbiagele B, Starkman S, Teal P, Lyden P, Kaste M, Davis SM, et al. Serum calcium as prognosticator in ischemic stroke. Stroke. 39:2231–2236. 2008.49. Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 89:857–866. 2016.50. Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 27:1641–1646. 1996.51. Park JH, Cho JH, Ahn JH, Choi SY, Lee TK, Lee JC, et al. Neuronal loss and gliosis in the rat striatum subjected to 15 and 30 minutes of middle cerebral artery occlusion. Metab Brain Dis. 33:775–784. 2018.52. Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 4:e4764. 2009.53. Reglodi D, Tamás A, Somogyvári-Vigh A, Szántó Z, Kertes E, Lénárd L, et al. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 23:2227–2234. 2002.54. Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 277:3310–3317. 2002.55. Santos AI, Carreira BP, Nobre RJ, Carvalho CM, Araújo IM. Stimulation of neural stem cell proliferation by inhibition of phosphodiesterase 5. Stem Cells Int. 2014:878397. 2014.56. Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl). 94:491–495. 1988.57. Scotto C, Deloulme JC, Rousseau D, Chambaz E, Baudier J. Calcium and S100B regulation of p53-dependent cell growth arrest and apoptosis. Mol Cell Biol. 18:4272–4281. 1998.58. Selinfreund RH, Barger SW, Pledger WJ, Van Eldik LJ. Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc Natl Acad Sci U S A. 88:3554–3558. 1991.59. Shin J, Gireesh G, Kim SW, Kim DS, Lee S, Kim YS, et al. Phospholipase C beta 4 in the medial septum controls cholinergic theta oscillations and anxiety behaviors. J Neurosci. 29:15375–15385. 2009.60. Siket MS. Treatment of acute ischemic stroke. Emerg Med Clin North Am. 34:861–882. 2016.61. Son Y, Kim K, Cho HR. Sildenafil protects neuronal cells from mitochondrial toxicity induced by β-amyloid peptide via ATP-sensitive K+ channels. Biochem Biophys Res Commun. 500:504–510. 2018.62. Steiner E, Enzmann GU, Lin S, Ghavampour S, Hannocks MJ, Zuber B, et al. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia. 60:1646–1659. 2012.63. Stephens S, Kenny RA, Rowan E, Allan L, Kalaria RN, Bradbury M, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 19:1053–1057. 2004.64. Tóthová L, Bábíčková J, Borbélyová V, Filová B, Šebeková K, Hodosy J. Chronic renal insufficiency does not induce behavioral and cognitive alteration in rats. Physiol Behav. 138:133–140. 2015.65. Teich AF, Sakurai M, Patel M, Holman C, Saeed F, Fiorito J, et al. PDE5 exists in human neurons and is a viable therapeutic target for neurologic disease. J Alzheimers Dis. 52:295–302. 2016.66. Tzoumas N, Farrah TE, Dhaun N, Webb DJ. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br J Pharmacol. 177:5467–5488. 2020.67. Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 37:550–555. 2006.68. Volke V, Soosaar A, Kõks S, Bourin M, Männistö PT, Vasar E. 7-Nitroindazole, a nitric oxide synthase inhibitor, has anxiolytic-like properties in exploratory models of anxiety. Psychopharmacology (Berl). 131:399–405. 1997.69. Volke V, Wegener G, Vasar E. Augmentation of the NO-cGMP cascade induces anxiogenic-like effect in mice. J Physiol Pharmacol. 54:653–660. 2003.70. Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 86:342–367. 2008.71. Yaghi S, Willey JZ, Khatri P. Minor ischemic stroke: triaging, disposition, and outcome. Neurol Clin Pract. 6:157–163. 2016.72. Yen TL, Hsu CK, Lu WJ, Hsieh CY, Hsiao G, Chou DS, et al. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J Agric Food Chem. 60:1937–1944. 2012.73. Yousuf S, Atif F, Ahmad M, Hoda N, Ishrat T, Khan B, et al. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 1250:242–253. 2009.74. Yu YH, Lee K, Sin DS, Park KH, Park DK, Kim DS. Altered functional efficacy of hippocampal interneuron during epileptogenesis following febrile seizures. Brain Res Bull. 131:25–38. 2017.75. Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, et al. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke. 36:847–852. 2005.76. Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 33:2675–2680. 2002.77. Zhu L, Yang JY, Xue X, Dong YX, Liu Y, Miao FR, et al. A novel phosphodiesterase-5 inhibitor: yonkenafil modulates neurogenesis, gliosis to improve cognitive function and ameliorates amyloid burden in an APP/PS1 transgenic mice model. Mech Ageing Dev. 150:34–45. 2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Movement Disorders Following Cerebrovascular Lesion in the Basal Ganglia Circuit
- Time-dependent Changes in Erectile Function and Responsiveness to Phosphodiesterase Type 5 Inhibitor in Streptozotocin-induced Diabetic Rats
- The Effect of Phosphodiesterase-4-Specific Inhibitor in the Rat Model of Spinal Nerve Ligation
- Induced Hypertension Using Phenylephrine in Patients with Acute Ischemic Stroke: A Case Report