Intest Res.
2022 Jul;20(3):329-341. 10.5217/ir.2021.00032.
Real-world data for golimumab treatment in patients with ulcerative colitis in Japan: interim analysis in post-marketing surveillance
- Affiliations
-
- 1Department of Internal Medicine II, Osaka Medical College Hospital, Takatsuki, Japan
- 2Janssen Pharmaceutical K.K., Tokyo, Japan
- 3Inflammatory Bowel Disease Center, Toho University Sakura Medical Center, Sakura, Japan
- KMID: 2531987
- DOI: http://doi.org/10.5217/ir.2021.00032
Abstract
- Background/Aims
Golimumab (GLM) is an anti-tumor necrosis factor-α drug approved for treating moderate-to-severe active ulcerative colitis (UC). A 52-week post-marketing surveillance (PMS) was initiated to evaluate its safety and effectiveness in patients with UC in Japan. We present an interim report of the ongoing PMS.
Methods
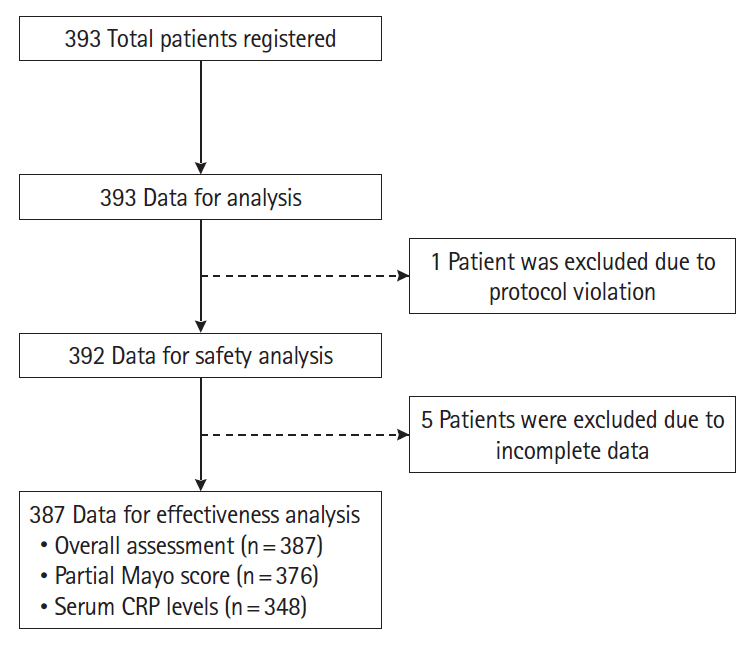
Patients received 200 mg of subcutaneous GLM at week 0, 100 mg at week 2, and 100 mg 4 weekly thereafter. The safety analysis set included 392 patients with UC, and the effectiveness analysis set 387 patients. Safety and effectiveness were assessed at week 6.
Results
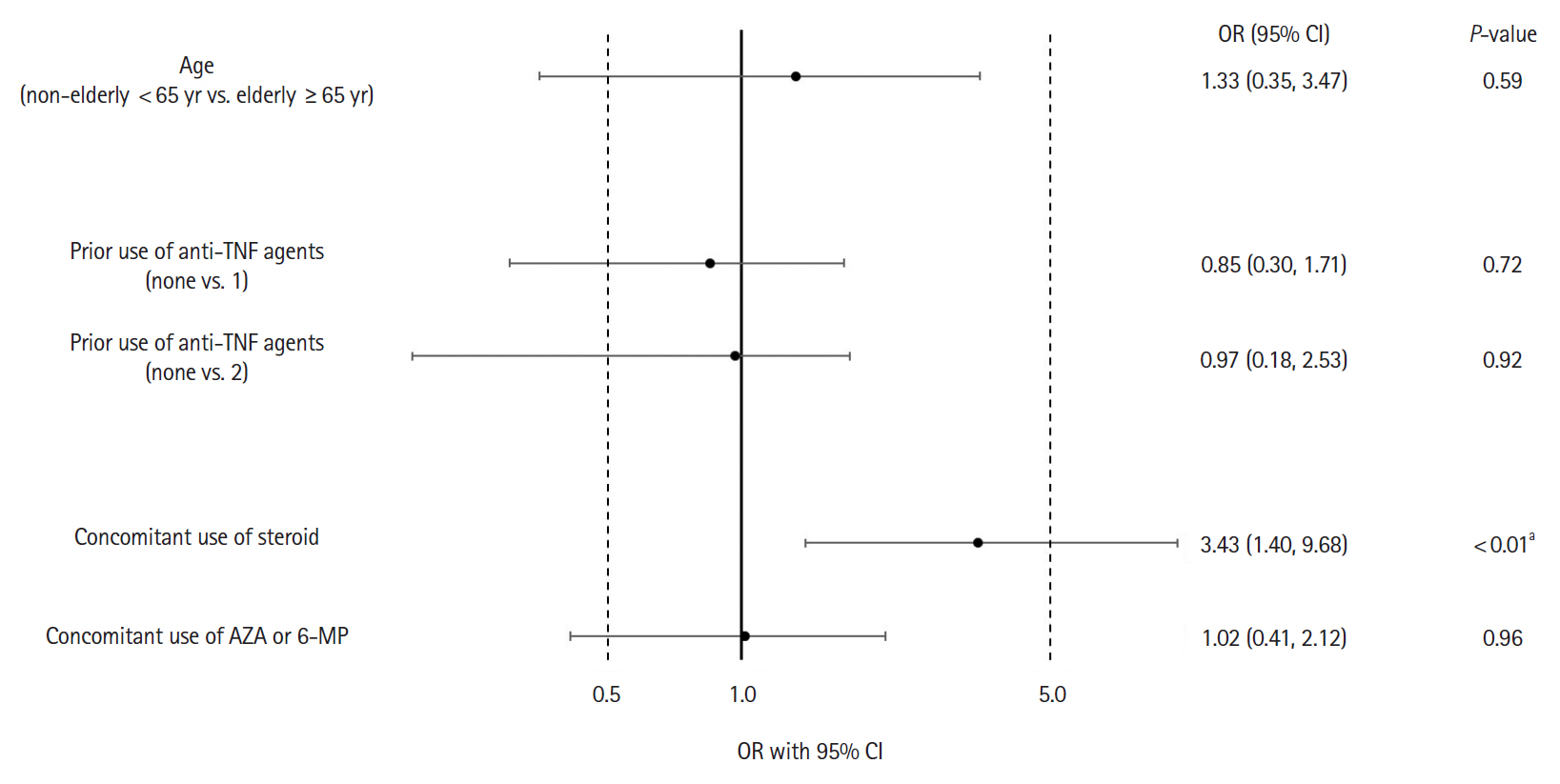
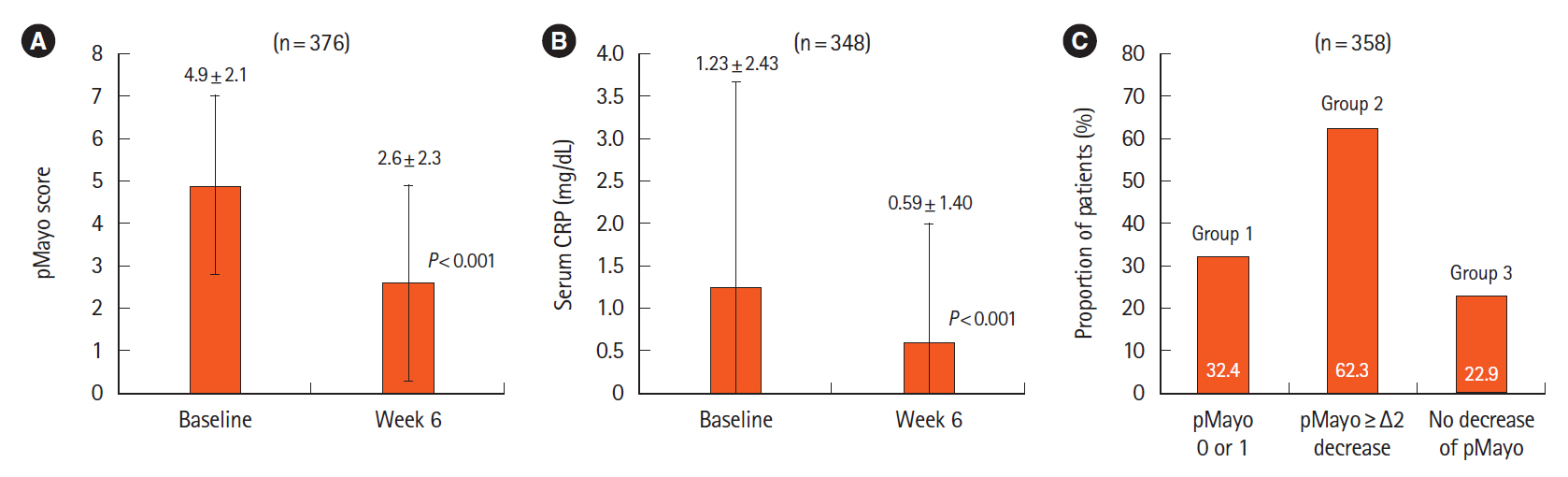
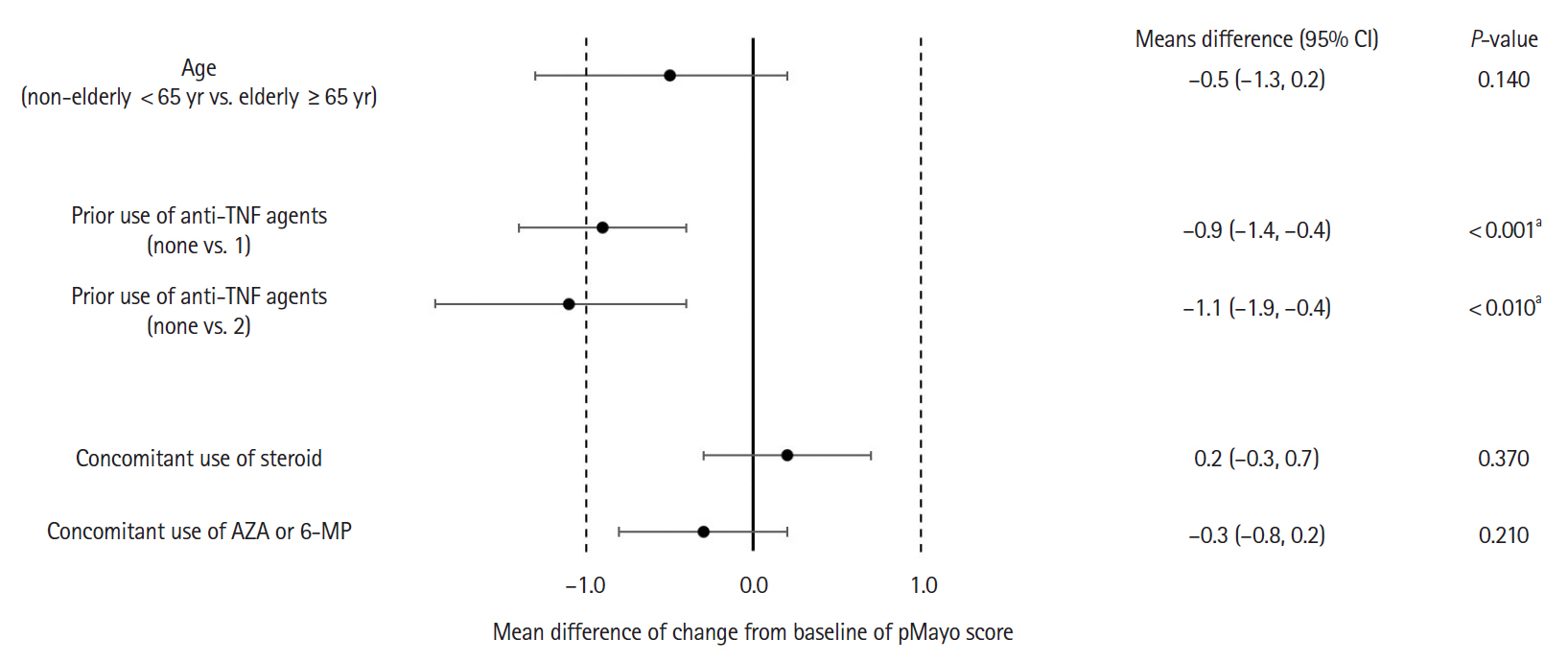
Adverse drug reactions (ADRs) were reported in 8.2% (32/392) and serious ADRs in 4.6% (18/392). The most frequent ADRs were infection and infestation (3.3%), with herpes zoster being the most common. ADRs were significantly higher in patients with concomitant corticosteroid use (odds ratio [OR], 3.45; 95% confidence interval [CI], 1.40–9.68). No significant difference in ADR incidence was observed between patients aged ≥65 and <65 years (OR, 1.23; 95% CI, 0.35–3.47). Six-week effectiveness of GLM was confirmed by a decrease in the partial Mayo score (–2.3; 95% CI, –2.6 to –2.1) and C-reactive protein levels (–0.64; 95% CI, –0.92 to –0.36), including in the biologics-experienced population.
Conclusions
The safety and effectiveness of GLM at week 6 in a real-world setting were demonstrated in patients with UC in Japan. ADR patterns were consistent with previous reports with no new safety signals. Concomitant corticosteroid use may be associated with increased ADR incidence. The final results of the ongoing PMS are necessary for further evaluation.
Keyword
Figure
Cited by 1 articles
-
Reviewing not Homer’s
Iliad , but “Kai Bao Ben Cao ”: indigo dye—the past, present, and future
Yusuke Yoshimatsu, Tomohisa Sujino, Takanori Kanai
Intest Res. 2023;21(2):174-176. doi: 10.5217/ir.2022.00018.
Reference
-
1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article2. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020; 6:74.
Article3. Ueno F, Nakayama Y, Hagiwara E, Kurimoto S, Hibi T. Impact of inflammatory bowel disease on Japanese patients’ quality of life: results of a patient questionnaire survey. J Gastroenterol. 2017; 52:555–567.
Article4. Yarlas A, Rubin DT, Panés J, et al. Burden of ulcerative colitis on functioning and well-being: a systematic literature review of the SF-36® health survey. J Crohns Colitis. 2018; 12:600–609.
Article5. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019; 50:992–1006.
Article6. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article7. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1465–1496.
Article8. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article9. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article10. Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-α agents: comparison among therapeutic TNF-α antagonists. Cytokine. 2018; 101:56–63.
Article11. Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016; 22:9300–9313.
Article12. Shealy DJ, Cai A, Staquet K, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. MAbs. 2010; 2:428–439.
Article13. Kay J, Matteson EL, Dasgupta B, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008; 58:964–975.
Article14. Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009; 60:976–986.
Article15. Inman RD, Davis JC Jr, Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008; 58:3402–3412.
Article16. Brunner HI, Ruperto N, Tzaribachev N, et al. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised-withdrawal trial. Ann Rheum Dis. 2018; 77:21–29.
Article17. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95.
Article18. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:96–109.
Article19. Hibi T, Imai Y, Senoo A, Ohta K, Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J Gastroenterol. 2017; 52:1101–1111.
Article20. Vermeire S, D’heygere F, Nakad A, et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 2018; 12:1193–1202.
Article21. Dragoni G, Le Grazie M, Orlandini B, Rogai F. Golimumab in inflammatory bowel diseases: present and future scenarios. Clin J Gastroenterol. 2019; 12:1–9.
Article22. Probert CS, Sebastian S, Gaya DR, et al. Golimumab induction and maintenance for moderate to severe ulcerative colitis: results from GO-COLITIS (Golimumab: a phase 4, UK, open label, single arm study on its utilization and impact in ulcerative Colitis). BMJ Open Gastroenterol. 2018; 5:e000212.23. Olivera P, Danese S, Pouillon L, Bonovas S, Peyrin-Biroulet L. Effectiveness of golimumab in ulcerative colitis: a review of the real world evidence. Dig Liver Dis. 2019; 51:327–334.
Article24. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.
Article25. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article26. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.27. Higashiyama M, Sugita A, Koganei K, et al. Management of elderly ulcerative colitis in Japan. J Gastroenterol. 2019; 54:571–586.
Article28. Bossa F, Biscaglia G, Valvano MR, et al. Real-life effectiveness and safety of golimumab and its predictors of response in patients with ulcerative colitis. Dig Dis Sci. 2020; 65:1767–1776.
Article29. Orlandini B, Dragoni G, Variola A, et al. Clinical efficacy and safety of golimumab in biologically experienced and naïve patients with active ulcerative colitis: a real-life experience from two Italian IBD centers. J Dig Dis. 2018; 19:468–474.
Article30. Tursi A, Allegretta L, Buccianti N, et al. Effectiveness and safety of golimumab in treating outpatient ulcerative colitis: a real-life prospective, multicentre, observational study in primary inflammatory bowel diseases centers. J Gastrointestin Liver Dis. 2017; 26:239–244.
Article31. Simponi Interview Form registered in PMDA search system for prescription drugs [Internet]. c2020. [cited 2020 Sep 25]. https://www.info.pmda.go.jp/downfiles/guide/ph/800155_3999433G1024_1_00G.pdf.32. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 37:420–429.
Article33. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018; 24:2258–2265.
Article34. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006; 55:420–426.
Article35. Morin C, Fardet L. Systemic glucocorticoid therapy: risk factors for reported adverse events and beliefs about the drug: a cross-sectional online survey of 820 patients. Clin Rheumatol. 2015; 34:2119–2126.
Article36. Singh S, Facciorusso A, Dulai PS, Jairath V, Sandborn WJ. Comparative risk of serious infections with biologic and/or immunosuppressive therapy in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:69–81.
Article37. Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008; 134:929–936.
Article38. Seddik M, Meliez H, Seguy D, Viget N, Cortot A, Colombel JF. Pneumocystis jiroveci (carinii) pneumonia following initiation of infliximab and azathioprine therapy in a patient with Crohn’s disease. Inflamm Bowel Dis. 2004; 10:436–437.
Article39. Kojima K, Sato T, Uchino M, et al. Clinical characteristics and risk factors for pneumocystis jirovecii pneumonia during immunosuppressive treatment in patients with ulcerative colitis: a retrospective study. J Gastrointestin Liver Dis. 2020; 29:167–173.
Article40. Ananthakrishnan AN, Donaldson T, Lasch K, Yajnik V. Management of inflammatory bowel disease in the elderly patient: challenges and opportunities. Inflamm Bowel Dis. 2017; 23:882–893.41. Zammarchi I, Lanzarotto F, Cannatelli R, et al. Elderly-onset vs adult-onset ulcerative colitis: a different natural history? BMC Gastroenterol. 2020; 20:147.
Article42. Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011; 9:30–35.
Article43. Komoto S, Higashiyama M, Watanabe C, et al. Clinical differences between elderly-onset ulcerative colitis and non-elderly-onset ulcerative colitis: a nationwide survey data in Japan. J Gastroenterol Hepatol. 2018; 33:1839–1843.
Article44. Taxonera C, Rodríguez C, Bertoletti F, et al. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2017; 23:1394–1402.
Article45. Samaan MA, Pavlidis P, Digby-Bell J, et al. Golimumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol. 2018; 9:221–231.
Article46. Christensen KR, Steenholdt C, Brynskov J. Clinical outcome of adalimumab therapy in patients with ulcerative colitis previously treated with infliximab: a Danish single-center cohort study. Scand J Gastroenterol. 2015; 50:1018–1024.
Article47. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015; 41:613–623.
Article48. Gemayel NC, Rizzello E, Atanasov P, Wirth D, Borsi A. Dose escalation and switching of biologics in ulcerative colitis: a systematic literature review in real-world evidence. Curr Med Res Opin. 2019; 35:1911–1923.
Article49. Taxonera C, Iglesias E, Muñoz F, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior antiTNF use and efficacy of dose escalation. Dig Dis Sci. 2017; 62:481–490.
Article50. Feuerstein JD, Isaacs KL, Schneider Y, et al. Spotlight: management of moderate-to-severe ulcerative colitis. Gastroenterology. 2020; 158:1464.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Golimumab Therapy in Ulcerative Colitis
- Infliximab biosimilar CT-P13 is interchangeable with its originator for patients with inflammatory bowel disease in real world practice
- Case Analysis in Drug Approval by FDA/EMA Using Real-World Data/Real-World Evidence
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study