Intest Res.
2019 Oct;17(4):504-515. 10.5217/ir.2019.00030.
Infliximab biosimilar CT-P13 is interchangeable with its originator for patients with inflammatory bowel disease in real world practice
- Affiliations
-
- 1Department of Gastroenterology, Chiba University Hospital, Chiba, Japan.
- 2Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- 3Quality & Pharmacovigilance Division, Pharmaceuticals Group, Nippon Kayaku Co., Ltd., Tokyo, Japan. kiyohiro.nishikawa@nipponkayaku.co.jp
- 4Department of Gastroenterology, Tane General Hospital, Osaka, Japan.
- 5Department of Internal Medicine, Sameshima Hospital, Kagoshima, Japan.
- 6Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan.
- 7Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan.
- KMID: 2465816
- DOI: http://doi.org/10.5217/ir.2019.00030
Abstract
- BACKGROUND/AIMS
An interim analysis of post-marketing surveillance of CT-P13, an infliximab biosimilar, was performed to evaluate its safety and efficacy in Japanese patients with inflammatory bowel disease.
METHODS
Patients were prospectively enrolled between November 2014 and March 2017, after the launch of CT-P13 in Japan, and case report forms of patients followed for at least 4 months were analyzed as of July 2018.
RESULTS
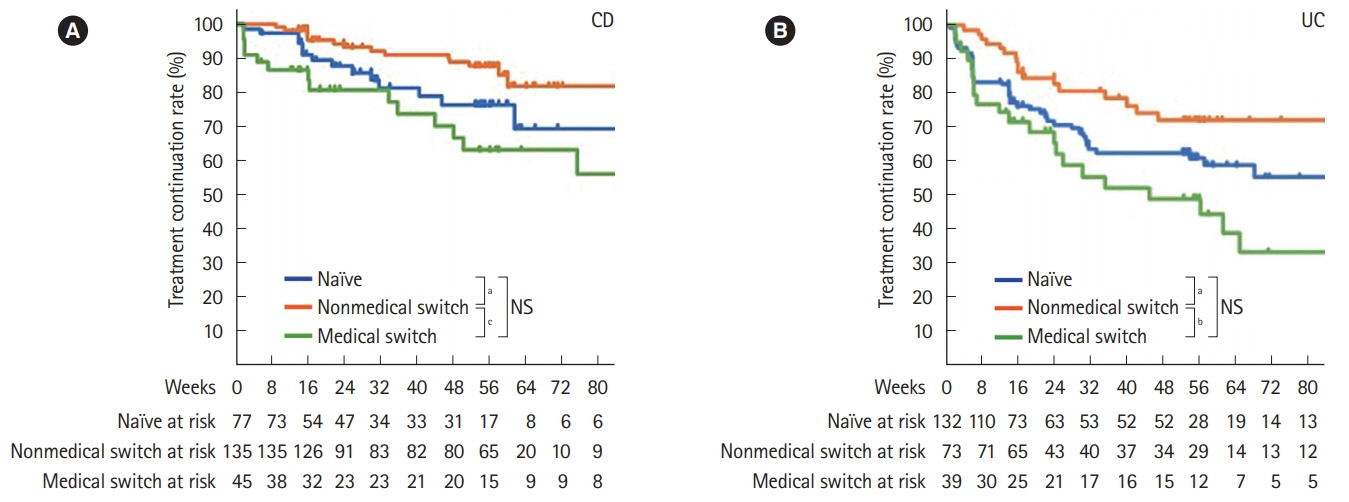
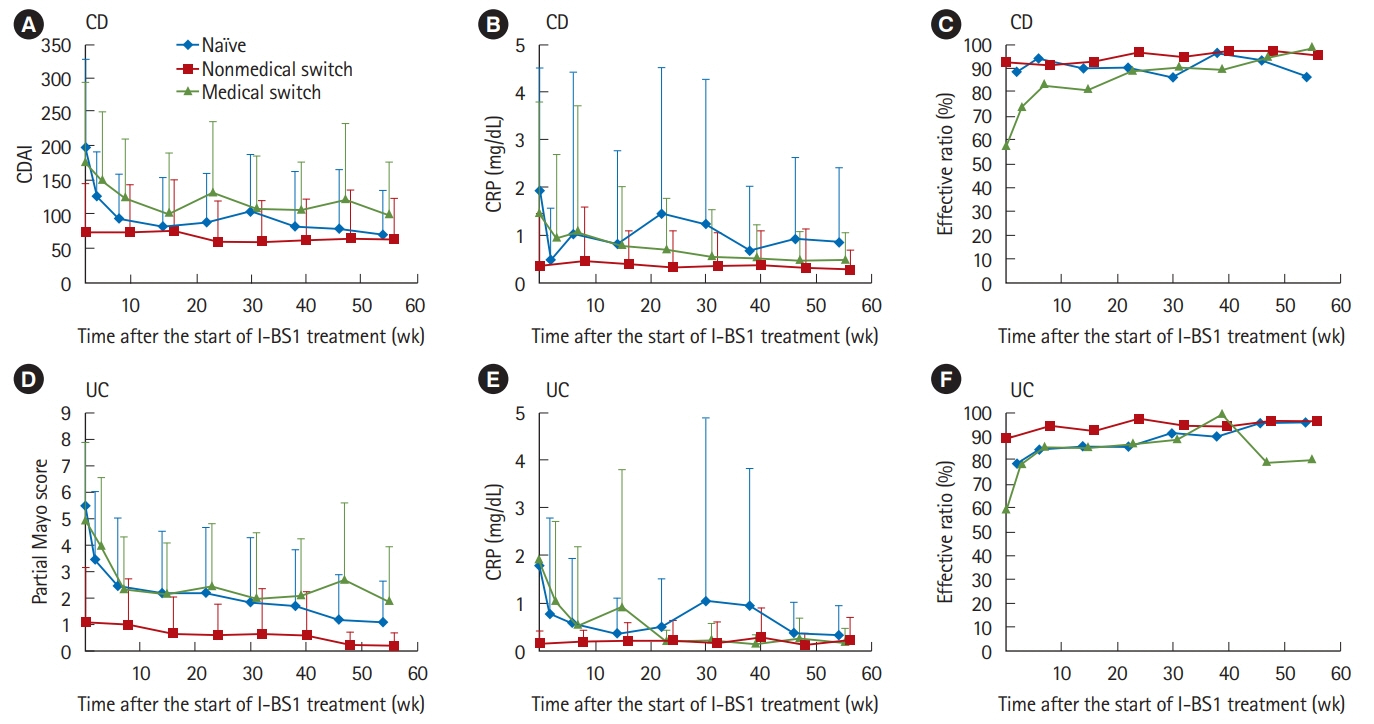
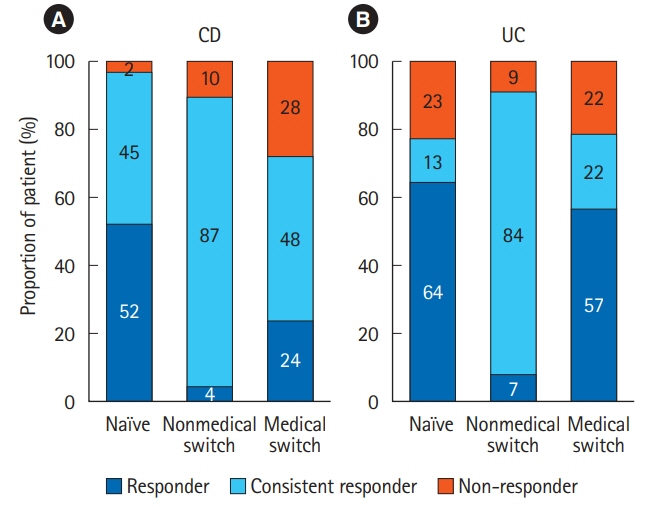
Of 523 patients in the analysis set, 372 remained on CT-P13 therapy, while 54 (20.2%) of 267 patients with Crohn's disease, and 97 (37.9%) of 256 patients with ulcerative colitis were withdrawn during follow-up. A total of 144 adverse drug reactions (ADRs) were reported in 106 patients (20.3%). Infusion reaction was the most frequent ADR observed in 49 patients (9.4%). Efficacy parameters decreased immediately after the start of treatment in naïve patients to anti-tumor necrosis factor-α antibody. In the patients switched from originator infliximab for nonmedical reasons, the decreased parameters due to proceeded treatment with the originator were maintained in low ranges, and the treatment continuation rate was high with low ADR incidence. In contrast, in patients switched for medical reasons such as adverse event or loss of response, the incidence of ADRs was high. However, the efficacy parameters were improved, and the treatment continuation rate was not significantly different from that of the naïve patient group.
CONCLUSIONS
In this interim analysis, CT-P13 was comparable to the originator infliximab with respect to ADRs and efficacy, and is therefore considered to be a cost-efficient interchangeable biosimilar for Japanese patients with inflammatory bowel disease.
MeSH Terms
Figure
Reference
-
1. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008; 117:244–279.2. Guo Y, Lu N, Bai A. Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: a recent update. Biomed Res Int. 2013; 2013:581631.
Article3. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013; 72:1605–1612.4. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013; 72:1613–1620.5. Takeuchi T, Yamanaka H, Tanaka Y, et al. Evaluation of the pharmacokinetic equivalence and 54-week efficacy and safety of CT-P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2015; 25:817–824.6. Hashikata H, Harada KH, Kagimura T, Nakamura M, Koizumi A. Usefulness of a large automated health records database in pharmacoepidemiology. Environ Health Prev Med. 2011; 16:313–319.
Article7. Molander P, Färkkilä M, Salminen K, et al. Outcome after discontinuation of TNFalpha-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis. 2014; 20:1021–1028.8. Casanova MJ, Chaparro M, García-Sánchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. 2017; 112:120–131.9. Kennedy NA, Warner B, Johnston EL, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther. 2016; 43:910–923.10. Remicade Interview Form registered in PMDA search system for prescription drugs. https://www.pmda.go.jp/PmdaSearch/iyakuSearch. Accessed January 2, 2019.11. Rossi RE, Parisi I, Despott EJ, et al. Anti-tumour necrosis factor agent and liver injury: literature review, recommendations for management. World J Gastroenterol. 2014; 20:17352–17359.12. Koller T, Galambosova M, Filakovska S, et al. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol. 2017; 23:4102–4111.
Article13. Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010; 7:15–29.14. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.15. Farkas K, Rutka M, Golovics PA, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016; 10:1273–1278.
Article16. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis. 2016; 10:133–140.17. Keil R, Wasserbauer M, Zádorová Z, et al. Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. 2016; 51:1062–1068.
Article18. Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Effectiveness and safety of CT-P13 (Biosimilar Infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci. 2017; 62:1305–1312.
Article19. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017; 23:233–243.20. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017; 389:2304–2316.21. Ratnakumaran R, To N, Gracie DJ, et al. Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand J Gastroenterol. 2018; 53:700–707.
Article22. Park DI. Current status of biosimilars in the treatment of inflammatory bowel diseases. Intest Res. 2016; 14:15–20.
Article23. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015; 21:182–197.
Article24. Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014; 63:1258–1264.
Article25. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015; 64:1539–1545.26. Pariente B, Pineton de Chambrun G, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:1199–1206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of biosimilars in the treatment of inflammatory bowel diseases
- Early Infliximab Trough Levels Predict the Long-term Efficacy of Infliximab in a Randomized Controlled Trial in Patients with Active Crohn’s Disease Comparing, between CT-P13 and Originator Infliximab
- Population Pharmacokinetic Model for the Use of Intravenous or Subcutaneous Infliximab in Patients with Inflammatory Bowel Disease: Real-World Data from a Prospective Cohort Study
- Knowledge and Viewpoints on Biosimilar Monoclonal Antibodies among Asian Physicians: Comparison with European Physicians
- Factors associated with anti-drug antibody production in ankylosing spondylitis patients treated with the infliximab biosimilar CT-P13