Korean J Gastroenterol.
2019 Dec;74(6):333-340. 10.4166/kjg.2019.74.6.333.
Knowledge and Viewpoints on Biosimilar Monoclonal Antibodies among Asian Physicians: Comparison with European Physicians
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. diksmc.park@samsung.com
- 2Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea.
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2469908
- DOI: http://doi.org/10.4166/kjg.2019.74.6.333
Abstract
- BACKGROUND/AIMS
Current knowledge and viewpoints regarding biosimilars among physicians in Asia are unknown, even though these were investigated by European Crohn's and Colitis Organization (ECCO) members in 2013 and 2015. Thus, this study conducted a multinational survey to assess the awareness of biosimilar monoclonal antibodies among Asian physicians.
METHODS
A 17-question multiple-choice anonymous web survey was conducted with the logistic support of the Asian Organization of Crohn's and Colitis (AOCC). Randomly selected AOCC members were invited by e-mail to participate between February 24, 2017 and March 26, 2017.
RESULTS
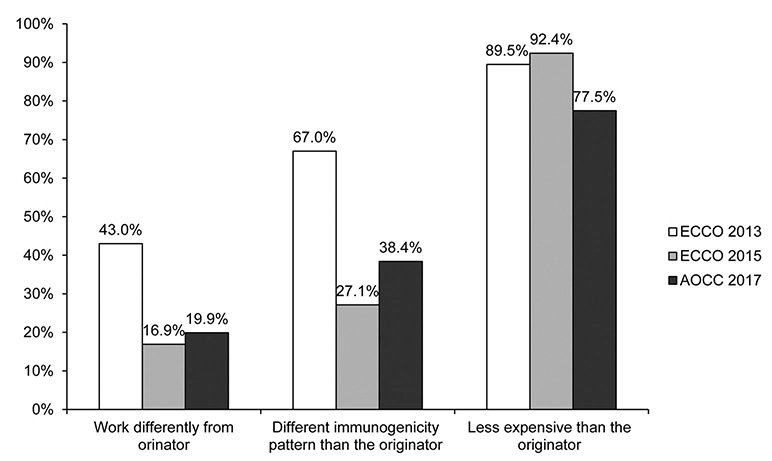
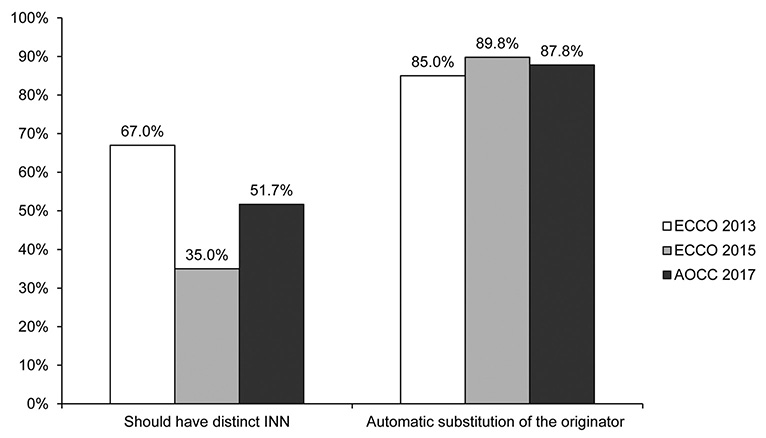
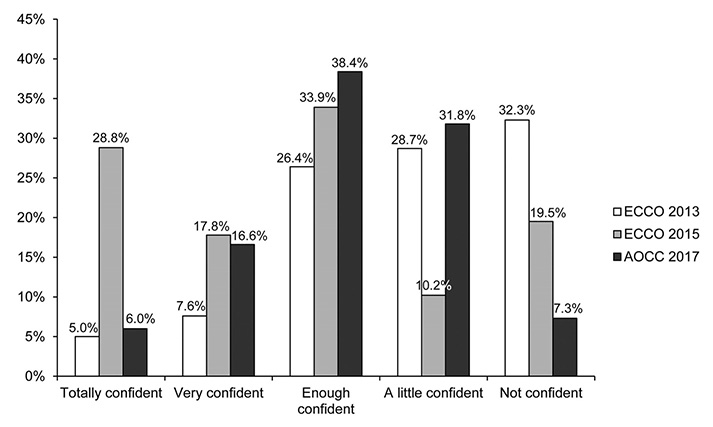
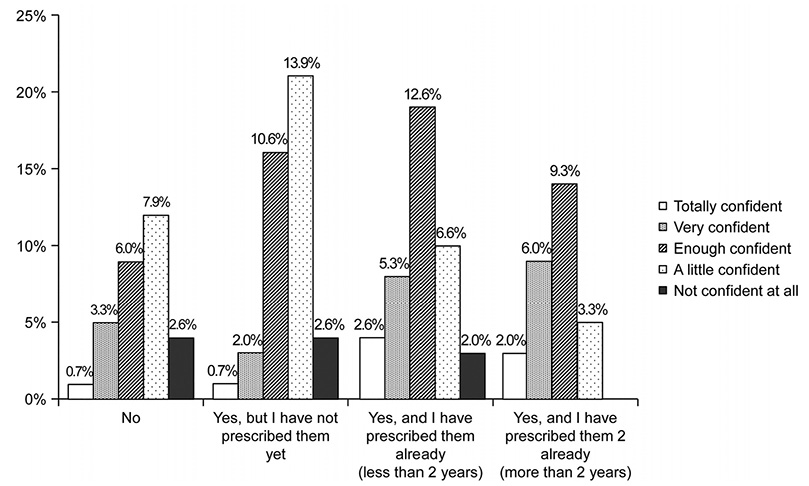
In total, 151 physicians from eight Asian countries responded to the survey. Most of the participants were gastroenterologists (96.6%), and 77.5% had cared for inflammatory bowel diseases (IBD) patients for more than 5 years. The majority of the respondents (66.2%) were aware that a biosimilar is similar but not equivalent to the originator. The majority of respondents (77.5%) considered cost saving to be the main advantage of biosimilars, but a high percentage of respondents (38.4%) were concerned about a different immunogenicity from that of the originator (92.4% and 27.1% respectively in ECCO 2015). Only 19.2% considered that the originator and biosimilars were interchangeable, and only 6.0% felt very confident in the use of biosimilars (44.4% and 28.8% respectively in ECCO 2015).
CONCLUSIONS
Asian gastroenterologists in 2017 are generally well informed about biosimilars. On the other hand, compared to the ECCO members surveyed in 2015, Asian gastroenterologists had more concerns and less confidence about the use of biosimilars in clinical practice. Thus, IBD-specific data on the comparison of the efficacy, safety, and immunogenicity in Asian patients are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Katsanos KH, Papadakis KA. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver. 2017; 11:455–463.2. Park DI. Current status of biosimilars in the treatment of inflammatory bowel diseases. Intest Res. 2016; 14:15–20.3. Danese S, Fiorino G, Michetti P. Viewpoint: knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn's and Colitis Organization. J Crohns Colitis. 2014; 8:1548–1550.4. Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn's Colitis Organization [ECCO] members: an updated survey. J Crohns Colitis. 2016; 10:1362–1365.5. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–2778.6. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.7. Ooi CJ, Makharia GK, Hilmi I, et al. Asia pacific consensus statements on Crohn's disease. Part 1: definition, diagnosis, and epidemiology: (Asia pacific Crohn's disease consensus--part 1). J Gastroenterol Hepatol. 2016; 31:45–55.8. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.9. Verghese NR, Barrenetxea J, Bhargava Y, Agrawal S, Finkelstein EA. Government pharmaceutical pricing strategies in the Asia-Pacific region: an overview. J Mark Access Health Policy. 2019; 7:1601060.10. Hasan SS, Kow CS, Dawoud D, Mohamed O, Baines D, Babar ZU. Pharmaceutical policy reforms to regulate drug prices in the Asia Pacific region: the case of Australia, China, India, Malaysia, New Zealand, and South Korea. Value Health Reg Issues. 2019; 18:18–23.11. Jung YS, Park DI, Kim YH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015; 30:1705–1712.12. Park SH, Kim YH, Lee JH, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015; 9 Suppl 1:35–44.13. Kim YH, Ye BD, Pesegova M, et al. Phase III randomized, double-blind, controlled trial to compare biosimilar infliximab (CT-P13) with innovator infliximab (INX) in patients with active Crohn's disease: early efficacy and safety results. Gastroenterology. 2017; 152:S65.14. Jahnsen J, Detlie TE, Vatn S, Ricanek P. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol. 2015; 9 Suppl 1:45–52.15. Keil R, Wasserbauer M, Zádorová Z, et al. Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 2016; 51:1062–1068.16. Smits LJ, Derikx LA, de Jong DJ, et al. Clinical outcomes following a switch from remicade® to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohns Colitis. 2016; 10:1287–1293.17. Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Effectiveness and safety of CT-P13 (biosimilar infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci. 2017; 62:1305–1312.18. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis. 2016; 10:133–140.19. Kaniewska M, Moniuszko A, Rydzewska G. The efficacy and safety of the biosimilar product (inflectra®) compared to the reference drug (remicade®) in rescue therapy in adult patients with ulcerative colitis. Prz Gastroenterol. 2017; 12:169–174.20. Farkas K, Rutka M, Golovics PA, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016; 10:1273–1278.21. Fiorino G, Ruiz-Argüello MB, Maguregui A, et al. Full interchangeability in regard to immunogenicity between the infliximab reference biologic and biosimilars CT-P13 and SB2 in inflammatory bowel disease. Inflamm Bowel Dis. 2018; 24:601–606.22. Smits LJT, Grelack A, Derikx LAAP, et al. Long-term clinical outcomes after switching from remicade® to biosimilar CT-P13 in inflammatory bowel disease. Dig Dis Sci. 2017; 62:3117–3122.23. Boone NW, Liu L, Romberg-Camps MJ, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol. 2018; 74:655–661.24. Schmitz EMH, Boekema PJ, Straathof JWA, et al. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther. 2018; 47:356–363.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation and Characterization of Anti - Human CTLA - 4 Monoclonal Antibodies
- Asian Physician's Perspectives on Biosimilars in Inflammatory Bowel Disease: Are We Ready to Use?
- Measurement of cyclosporine concentration in whole blood of renal transplant patients: comparison of cyclosporine concentrations determined by radioimmunoassay using specific and nonspecific monoclonal antibodies
- Physicians and Ethics
- Production and characterization of monoclonal antibodies to perchloric acid soluble antigen of M.tuberculosis and its clinical application