J Korean Neurosurg Soc.
2022 Jul;65(4):572-581. 10.3340/jkns.2021.0202.
Assessment and Comparison of Three Dimensional Exoscopes for Near-Infrared Fluorescence-Guided Surgery Using Second-Window Indocyanine-Green
- Affiliations
-
- 1Department of Neurosurgery, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
- 2Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
- KMID: 2531626
- DOI: http://doi.org/10.3340/jkns.2021.0202
Abstract
Objective
: Compared to microscopes, exoscopes have advantages in field-depth, ergonomics, and educational value. Exoscopes are especially well-poised for adaptation into fluorescence-guided surgery (FGS) due to their excitation source, light path, and image processing capabilities. We evaluated the feasibility of near-infrared FGS using a 3-dimensional (3D), 4 K exoscope with nearinfrared fluorescence imaging capability. We then compared it to the most sensitive, commercially-available near-infrared exoscope system (3D and 960 p). In-vitro and intraoperative comparisons were performed.
Methods
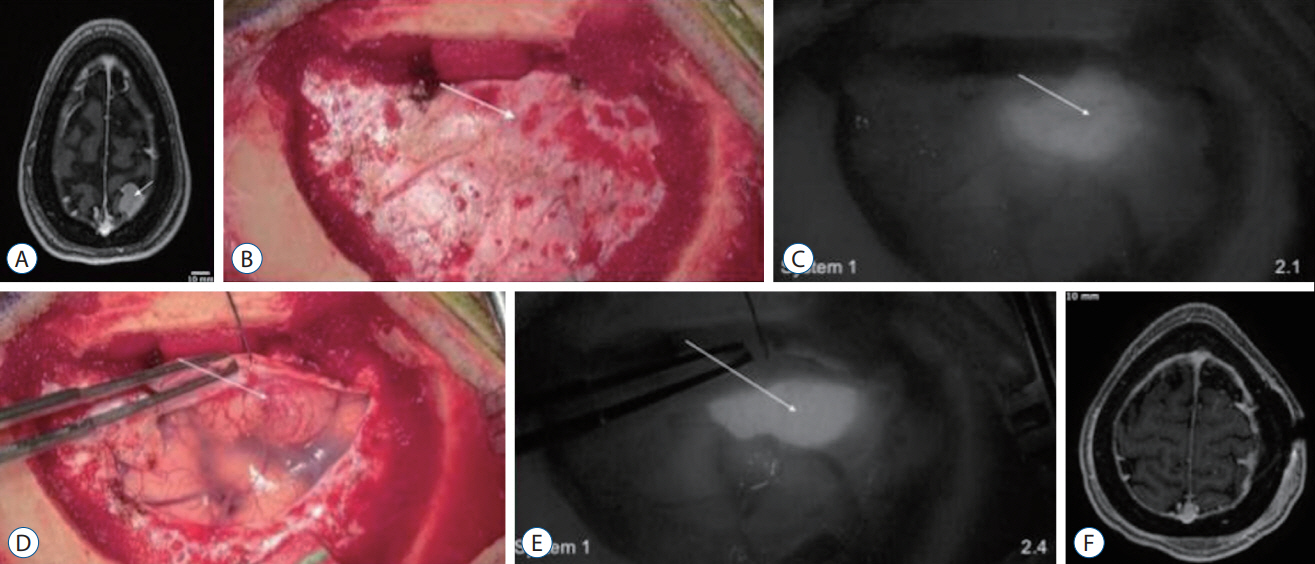
: Serial dilutions of indocyanine-green (1–2000 μg/mL) were imaged with the 3D, 4 K Olympus Orbeye (system 1) and the 3D, 960 p VisionSense Iridium (system 2). Near-infrared sensitivity was calculated using signal-to-background ratios (SBRs). In addition, three patients with brain tumors were administered indocyanine-green and imaged with system 1, with two also imaged with system 2 for comparison.
Results
: Systems 1 and 2 detected near-infrared fluorescence from indocyanine green concentrations of >250 μg/L and >31.3 μg/L, respectively. Intraoperatively, system 1 visualized strong near-infrared fluorescence from two, strongly gadoliniumenhancing meningiomas (SBR=2.4, 1.7). The high-resolution, bright images were sufficient for the surgeon to appreciate the underlying anatomy in the near-infrared mode. However, system 1 was not able to visualize fluorescence from a weakly-enhancing intraparenchymal metastasis. In contrast, system 2 successfully visualized both the meningioma and the metastasis but lacked high resolution stereopsis.
Conclusion
: Three-dimensional exoscope systems provide an alternative visualization platform for both standard microsurgery and near-infrared fluorescent guided surgery. However, when tumor fluorescence is weak (i.e., low fluorophore uptake, deep tumors), highly sensitive near-infrared visualization systems may be required.
Keyword
Figure
Reference
-
References
1. Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C, et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg Focus. 36:E5. 2014.
Article2. Belykh E, Miller EJ, Patel AA, Yazdanabadi MI, Martirosyan NL, Yağmurlu K, et al. Diagnostic accuracy of a confocal laser endomicroscope for in vivo differentiation between normal injured and tumor tissue during fluorescein-guided glioma resection: laboratory investigation. World Neurosurg. 115:e337–e348. 2018.3. Chakravarthy V, Sheikh S, Schmidt E, Steinmetz M. Imaging technologies in spine surgery. Neurosurg Clin N Am. 31:93–101. 2020.
Article4. Cho SS, Jeon J, Buch L, Nag S, Nasrallah M, Low PS, et al. Intraoperative near-infrared imaging with receptor-specific versus passive delivery of fluorescent agents in pituitary adenomas. J Neurosurg. 131:1974–1984. 2018.
Article5. Cho SS, Salinas R, De Ravin E, Teng CW, Li C, Abdullah KG, et al. Near-infrared imaging with second-window indocyanine green in newly diagnosed high-grade gliomas predicts gadolinium enhancement on postoperative magnetic resonance imaging. Mol Imaging Biol. 22:1427–1437. 2020.
Article6. Cho SS, Salinas R, Lee JYK. Indocyanine-green for fluorescence-guided surgery of brain tumors: evidence, techniques, and practical experience. Front Surg. 6:11. 2019.
Article7. Cho SS, Teng CW, Ramayya A, Buch L, Hussain J, Harsch J, et al. Surface-registration frameless stereotactic navigation is less accurate during prone surgeries: intraoperative near-infrared visualization using second window indocyanine green offers an adjunct. Mol Imaging Biol. 22:1572–1580. 2020.
Article8. Cho SS, Zeh R, Pierce JT, Salinas R, Singhal S, Lee JYK. Comparison of near-infrared imaging camera systems for intracranial tumor detection. Mol Imaging Biol. 20:213–220. 2018.
Article9. Connally R, Jin D, Piper J. High intensity solid-state UV source for time-gated luminescence microscopy. Cytometry A. 69:1020–1027. 2006.
Article10. DSouza AV, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt. 21:80901. 2016.
Article11. Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn Ther. 16:35–43. 2016.
Article12. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 63:136–151. 2011.
Article13. Hadjipanayis CG, Stummer W. 5-ALA and FDA approval for glioma surgery. J Neurooncol. 141:479–486. 2019.
Article14. Herlan S, Marquardt JS, Hirt B, Tatagiba M, Ebner FH. 3D exoscope system in neurosurgery-comparison of a standard operating microscope with a new 3D exoscope in the cadaver lab. Oper Neurosurg (Hagerstown). 17:518–524. 2019.
Article15. Kaibori M, Matsui K, Ishizaki M, Iida H, Okumura T, Sakaguchi T, et al. Intraoperative detection of superficial liver tumors by fluorescence imaging using indocyanine green and 5-aminolevulinic acid. Anticancer Res. 36:1841–1849. 2016.16. Keating J, Newton A, Venegas O, Nims S, Zeh R, Predina J, et al. Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann Thorac Surg. 103:390–398. 2017.
Article17. Kwan K, Schneider JR, Du V, Falting L, Boockvar JA, Oren J, et al. Lessons learned using a high-definition 3-dimensional exoscope for spinal surgery. Oper Neurosurg (Hagerstown). 16:619–625. 2019.
Article18. Lee JYK, Cho SS, Stummer W, Tanyi JL, Vahrmeijer AL, Rosenthal E, et al. Review of clinical trials in intraoperative molecular imaging during cancer surgery. J Biomed Opt. 24:1–8. 2019.
Article19. Li C, Buch L, Cho S, Lee JYK. Near-infrared intraoperative molecular imaging with conventional neurosurgical microscope can be improved with narrow band “boost” excitation. Acta Neurochir (Wien). 161:2311–2318. 2019.
Article20. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 41:189–207. 2001.
Article21. Murai Y, Sato S, Yui K, Morimoto D, Ozeki T, Yamaguchi M, et al. Preliminary clinical microneurosurgical experience with the 4K3-dimensional microvideoscope (ORBEYE) system for microneurological surgery: observation study. Oper Neurosurg (Hagerstown). 16:707–716. 2019.
Article22. Nishiyama K. From exoscope into the next generation. J Korean Neurosurg Soc. 60:289–293. 2017.
Article23. Pagoto A, Garello F, Marini GM, Tripepi M, Arena F, Bardini P, et al. Novel gastrin-releasing peptide receptor targeted near-infrared fluorescence dye for image-guided surgery of prostate cancer. Mol Imaging Biol. 22:85–93. 2020.
Article24. Ricciardi L, Chaichana KL, Cardia A, Stifano V, Rossini Z, Olivi A, et al. The exoscope in neurosurgery: an innovative “point of view”. A systematic review of the technical, surgical and educational aspects. World Neurosurg. 124:136–144. 2019.
Article25. Ricciardi L, Mattogno PP, Olivi A, Sturiale CL. Exoscope era: next technical and educational step in microneurosurgery. World Neurosurg. 128:371–373. 2019.
Article26. Rossini Z, Cardia A, Milani D, Lasio GB, Fornari M, D’Angelo V. VITOM 3D: preliminary experience in cranial surgery. World Neurosurg. 107:663–668. 2017.
Article27. Sack J, Steinberg JA, Rennert RC, Hatefi D, Pannell JS, Levy M, et al. Initial experience using a high-definition 3-dimensional exoscope system for microneurosurgery. Oper Neurosurg (Hagerstown). 14:395–401. 2018.
Article28. Samkoe KS, Sardar HS, Bates BD, Tselepidakis NN, Gunn JR, Hoffer-Hawlik KA, et al. Preclinical imaging of epidermal growth factor receptor with ABY-029 in soft-tissue sarcoma for fluorescence-guided surgery and tumor detection. J Surg Oncol. 199:1077–1086. 2019.
Article29. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7:392–401. 2006.
Article30. Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 74:310–320. discussion 319-320. 2014.31. Yasargil MG. Microneurosurgery, Vol 1: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. New York: Georg Thieme Verlag Stuttgart;1984.32. Yazaki P, Lwin T, Minnix M, Li L, Sherman A, Molnar J, et al. Improved antibody-guided surgery with a near-infrared dye on a pegylated linker for CEA-positive tumors. J Biomed Opt. 24:1–9. 2019.
Article33. Yu D, Green C, Kasten SJ, Sackllah ME, Armstrong TJ. Effect of alternative video displays on postures, perceived effort, and performance during microsurgery skill tasks. Appl Erg. 53:281–289. 2016.
Article34. Yu D, Sackllah M, Woolley C, Kasten S, Armstrong T. Quantitative posture analysis of 2D, 3D, and optical microscope visualization methods for microsurgery tasks. Work 41 Suppl. 1:1944–1947. 2012.
Article35. Zeh R, Sheikh S, Xia L, Pierce J, Newton A, Predina J, et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS One. 24:e0182034. 2017.
Article36. Zhang DY, Singhal S, Lee JYK. Optical principles of fluorescence-guided brain tumor surgery: a practical primer for the neurosurgeon. Neurosurgery. 85:312–324. 2019.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Utility of Indocyanine Green Fluorescence Imaging in Wound Assessment
- Structure-Inherent Targeting of Near-Infrared Fluorophores for Image-Guided Surgery
- Indocyanine green and near-infrared fluorescenceguided surgery for gastric cancer: a narrative review
- Indocyanine Green-Guided Video-Assisted Thoracoscopic Surgery for Resection of an Ectopic Mediastinal Parathyroid Adenoma
- Real-Time Localization of Parathyroid Glands with Near Infrared Light during Thyroid and Parathyroid Surgery