Ann Surg Treat Res.
2022 Jul;103(1):32-39. 10.4174/astr.2022.103.1.32.
Predicting stage ypT0–1N0 for nonradical management in patients with middle or low rectal cancer who undergo neoadjuvant chemoradiotherapy: a retrospective cohort study
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Radiology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2531145
- DOI: http://doi.org/10.4174/astr.2022.103.1.32
Abstract
- Purpose
It is important to discover predictive factors that can identify rectal cancer patients who will respond well to neoadjuvant concurrent chemoradiotherapy (CCRT) to develop management strategies, preserve sphincter and avoid overtreatment. This study explored clinical factors that would predict the adequacy of nonradical management after CCRT in patients with middle or low rectal cancer.
Methods
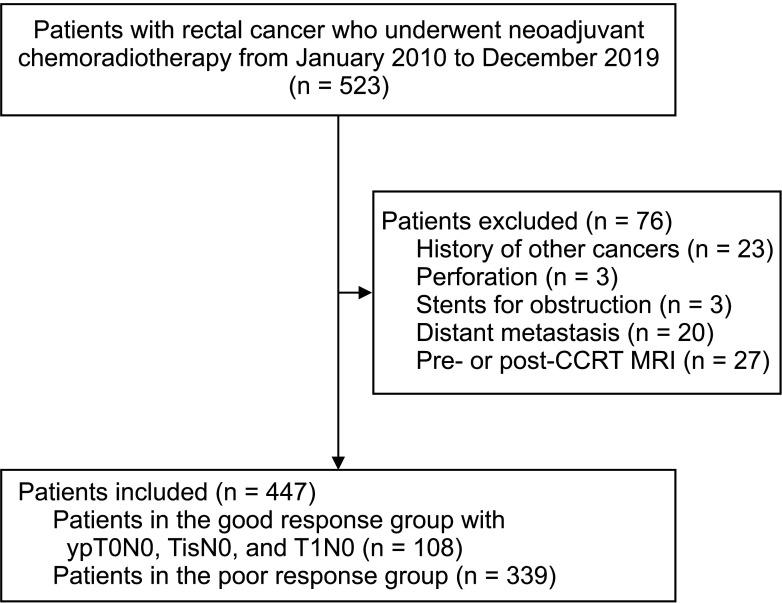
We retrospectively evaluated 447 patients with middle or low rectal cancer who were treated with curative surgery after neoadjuvant CCRT between January 2010 and December 2019. The good response group comprised patients with stages ypT0–1N0 on resection after CCRT; the remaining patients were included in the poor response group.
Results
Of 447 patients (mean age, 60.37 ± 11.85 years), 108 (24.2%) had ypT0–1N0 (71.3% with ypT0N0, 4.6% with ypTisN0, and 24.1% with ypT1N0). Overall, 19 patients with cT1–2 (50.0% vs. 21.8% with cT3–4, P < 0.001), 22 with well-differentiated tumors (51.2% vs. 21.3% with moderately/poorly differentiated tumors, P < 0.001), 16 with fungating tumors (47.1% vs.22.3% with other types, P = 0.001), and 66 with anterior/posterior circumference direction (28.9% vs. 19.2% with lateral/ encircling direction, P = 0.016) had stage ypT0–1N0. On multivariable analysis, cT1–2 (P = 0.021) and well-differentiated tumor (P = 0.001) were independent predictors of ypT0–1N0. Fungating tumors were not significantly associated with ypT0– 1N0 (P = 0.054).
Conclusion
Stage cT1–2 and well differentiation are predictors of ypT0–1N0, while fungating tumors could be considered clinically meaningful, possibly identifying candidates for nonradical treatment post-CCRT.
Figure
Cited by 1 articles
-
Weighing the benefits of lymphadenectomy in early-stage colorectal cancer
Seung Min Baik, Ryung-Ah Lee
Ann Surg Treat Res. 2023;105(5):245-251. doi: 10.4174/astr.2023.105.5.245.
Reference
-
1. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–844. PMID: 20692872.
Article2. Lim SB, Yu CS, Hong YS, Kim TW, Park JH, Kim JH, et al. Failure patterns correlate with the tumor response after preoperative chemoradiotherapy for locally advanced rectal cancer. J Surg Oncol. 2012; 106:667–673. PMID: 22688948.
Article3. Song C, Chung JH, Kang SB, Kim DW, Oh HK, Lee HS, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a proposal for a modified staging system. Cancers (Basel). 2018; 10:319.
Article4. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004; 240:711–718. PMID: 15383798.
Article5. National Comprehensive Cancer Network (NCCN). Rectal cancer, version 1.2021 clinical practice guidelines in oncology [Internet]. Plymouth Meeting, PA: NCCN;c2021. cited 2022 Feb 25. Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.6. Park CH, Kim HC, Cho YB, Yun SH, Lee WY, Park YS, et al. Predicting tumor response after preoperative chemoradiation using clinical parameters in rectal cancer. World J Gastroenterol. 2011; 17:5310–5316. PMID: 22219601.
Article7. Garland ML, Vather R, Bunkley N, Pearse M, Bissett IP. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis. 2014; 29:301–307. PMID: 24420737.
Article8. Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013; 56:698–703. PMID: 23652742.
Article9. Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2016; 23:1177–1186. PMID: 26668083.
Article10. Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007; 69:1167–1172. PMID: 17967307.
Article11. Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–8696. PMID: 16246976.
Article12. Park YA, Sohn SK, Seong J, Baik SH, Lee KY, Kim NK, et al. Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol. 2006; 93:145–150. PMID: 16425302.
Article13. Lee JH, Song C, Kang SB, Lee HS, Lee KW, Kim JS. Predicting pathological complete regression with haematological markers during neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Anticancer Res. 2018; 38:6905–6910. PMID: 30504408.
Article14. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(Suppl 4):iv22–iv40. PMID: 28881920.
Article15. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018; 16:874–901. PMID: 30006429.
Article16. Heo J, Chun M, Noh OK, Oh YT, Suh KW, Park JE, et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat. 2016; 48:232–239. PMID: 25779365.
Article17. Clarke TL, White DA, Osborne ME, Shaw AM, Smart NJ, Daniels IR. Predicting response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer with serum biomarkers. Ann R Coll Surg Engl. 2017; 99:373–377. PMID: 28462648.
Article18. Zhang WY, Chen XX, Chen WH, Zhang H, Zou CL. Mean corpuscular volume as a predictive factor of response to preoperative chemoradiotherapy in locally advanced rectal cancer. Gastroenterol Res Pract. 2018; 2018:6976375. PMID: 29743887.
Article19. Watanabe T, Kobunai T, Akiyoshi T, Matsuda K, Ishihara S, Nozawa K. Prediction of response to preoperative chemoradiotherapy in rectal cancer by using reverse transcriptase polymerase chain reaction analysis of four genes. Dis Colon Rectum. 2014; 57:23–31. PMID: 24316942.
Article20. Hur H, Tulina I, Cho MS, Min BS, Koom WS, Lim JS, et al. Biomarker-based scoring system for prediction of tumor response after preoperative chemoradiotherapy in rectal cancer by reverse transcriptase polymerase chain reaction analysis. Dis Colon Rectum. 2016; 59:1174–1182. PMID: 27824703.
Article21. You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020; 63:1191–1222. PMID: 33216491.
Article22. Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 2015; 107:djv002. PMID: 25667420.
Article23. Al-Husseini MJ, Saad AM, Mohamed HH, Alkhayat MA, Sonbol MB, Abdel-Rahman O. Impact of prior malignancies on outcome of colorectal cancer; revisiting clinical trial eligibility criteria. BMC Cancer. 2019; 19:863. PMID: 31470823.
Article24. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010; 11:637–645. PMID: 20610322.
Article25. Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015; 16:1537–1546. PMID: 26474521.
Article26. Fischer J, Eglinton TW, Frizelle FA. Clinical predictors of response to chemoradiotherapy for rectal cancer as an aid to organ preservation. ANZ J Surg. 2021; 91:1190–1195. PMID: 33404195.
Article27. Lorimer PD, Motz BM, Kirks RC, Boselli DM, Walsh KK, Prabhu RS, et al. Pathologic complete response rates after neoadjuvant treatment in rectal cancer: an analysis of the National Cancer Database. Ann Surg Oncol. 2017; 24:2095–2103. PMID: 28534080.
Article28. Guillem JG, Chessin DB, Shia J, Moore HG, Mazumdar M, Bernard B, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005; 23:3475–3479. PMID: 15908656.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oncologic comparison between nonradical management and total mesorectal excision in good responders after chemoradiotherapy in patients with mid-to-low rectal cancer
- Clinical characteristics of rectal cancer patients with neoadjuvant chemoradiotherapy: a nationwide population-based cohort study in South Korea
- Long-term bowel functional outcomes following anal sphincter-preserving surgery for upper and middle rectal cancer: a single-center longitudinal study
- Outcome of Local Excision Following Preoperative Chemoradiotherapy for Clinically T2 Distal Rectal Cancer: A Multicenter Retrospective Study (KROG 12-06)
- Clinical Implication of Lateral Pelvic Lymph Node Metastasis in Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy