Korean J Pain.
2022 Jul;35(3):291-302. 10.3344/kjp.2022.35.3.291.
Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: relevance to its antioxidant and anti-inflammatory activities
- Affiliations
-
- 1Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Medical Biology Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 4Research Center of Oils and Fats, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 5Department of Neuroscience, Faculty of Advanced Technologies in Medical Sciences, Iran University of Medical Sciences, Tehran, Iran
- 6Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- KMID: 2530984
- DOI: http://doi.org/10.3344/kjp.2022.35.3.291
Abstract
- Background
Spinal cord injury (SCI) is one of the most debilitating disorders throughout the world, causing persistent sensory-motor dysfunction, with no effective treatment. Oxidative stress and inflammatory responses play key roles in the secondary phase of SCI. Naringenin (NAR) is a natural flavonoid with known antiinflammatory and antioxidative properties. This study aims at evaluating the effects of intrathecal NAR administration on sensory-motor disability after SCI.
Methods
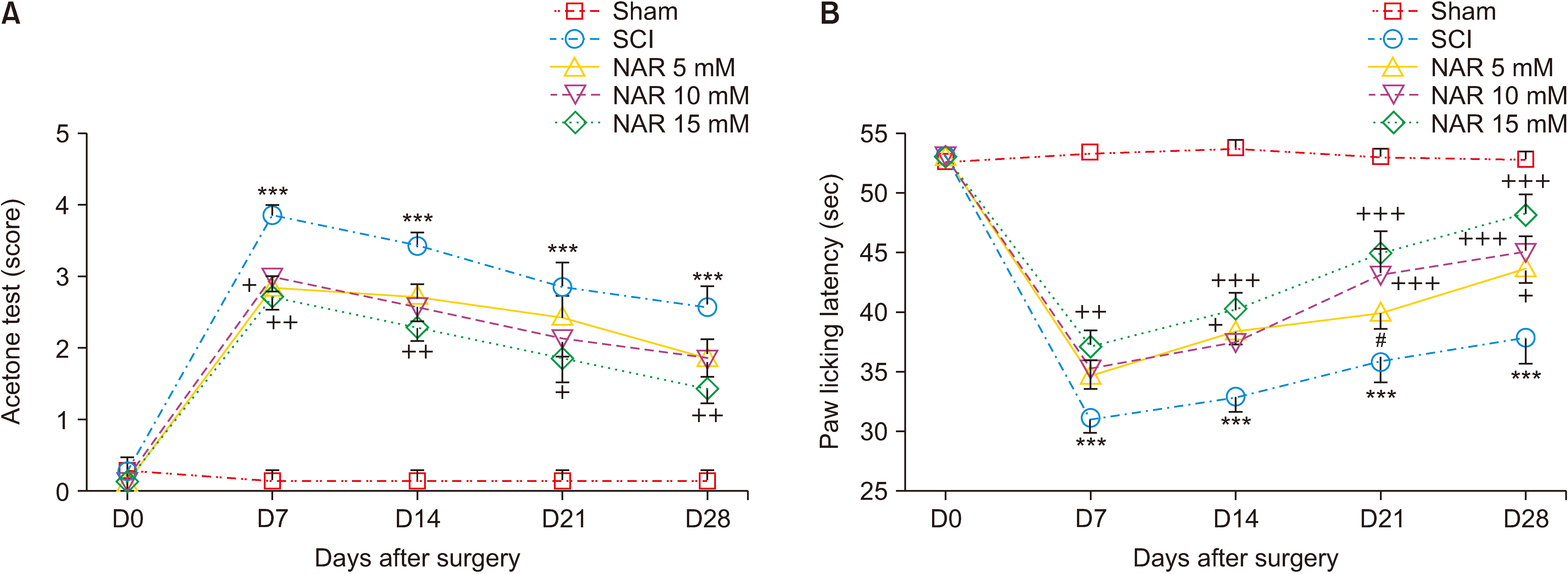
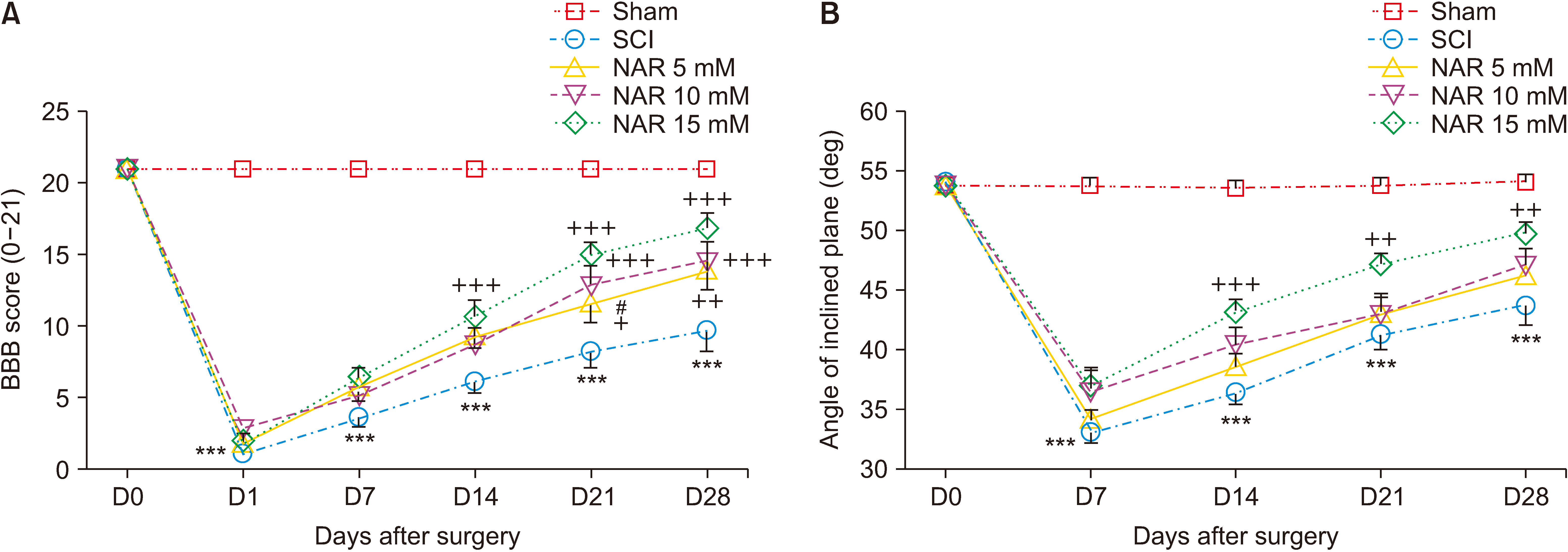
Animals underwent a severe compression injury using an aneurysm clip. About 30 minutes after surgery, NAR was injected intrathecally at the doses of 5, 10, and 15 mM in 20 µL volumes. For the assessment of neuropathic pain and locomotor function, acetone drop, hot plate, inclined plane, and Basso, Beattie, Bresnahan tests were carried out weekly till day 28 post-SCI. Effects of NAR on matrix metalloproteinase (MMP)-2 and MMP-9 activity was appraised by gelatin zymography. Also, histopathological analyses and serum levels of glutathione (GSH), catalase and nitrite were measured in different groups.
Results
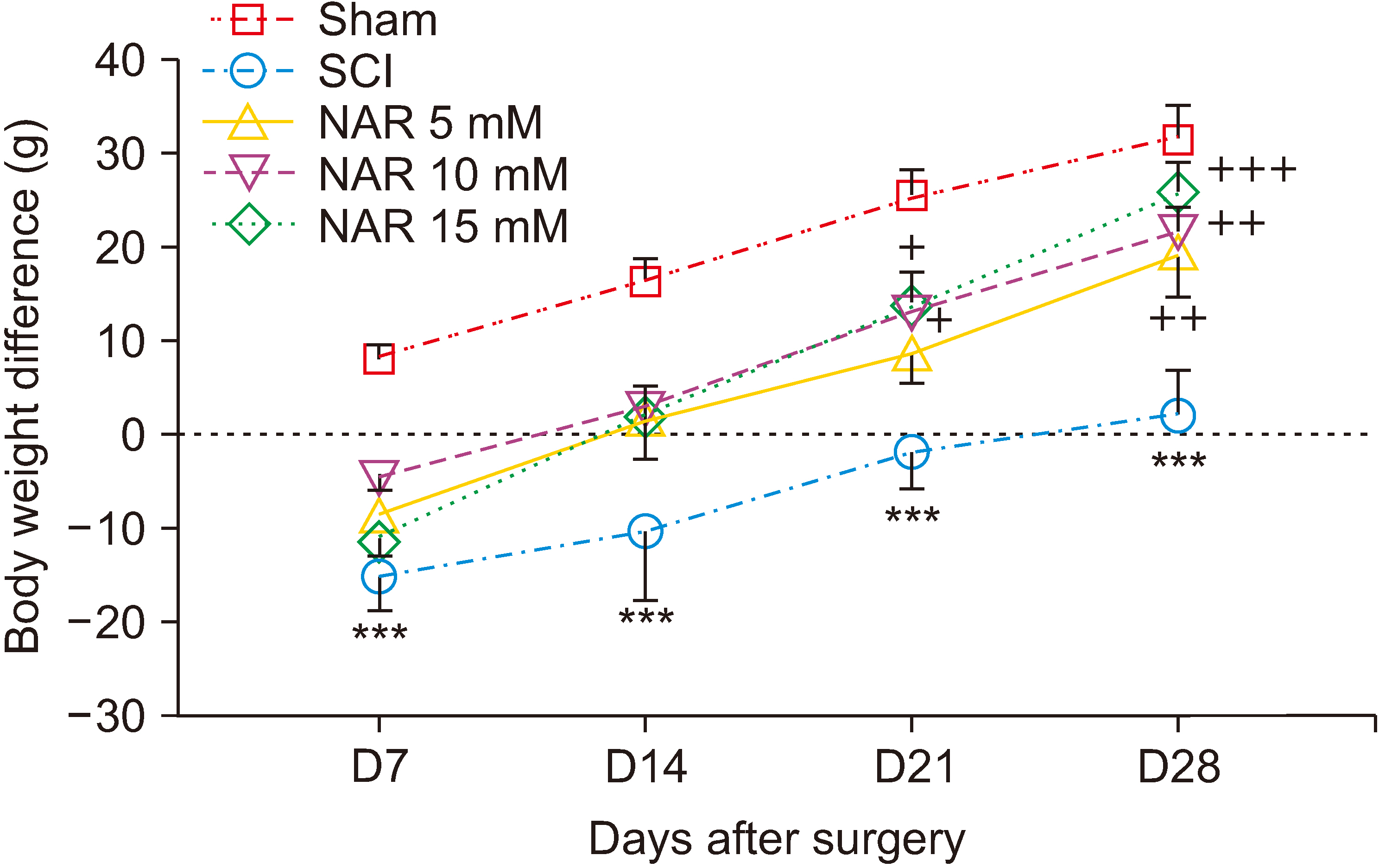
NAR reduced neuropathic pain, improved locomotor function, and also attenuated SCI-induced weight loss weekly till day 28 post-SCI. Zymography analysis showed that NAR suppressed MMP-9 activity, whereas it increased that of MMP-2, indicating its anti-neuroinflammatory effects. Also, intrathecal NAR modified oxidative stress related markers GSH, catalase, and nitrite levels. Besides, the neuroprotective effect of NAR was corroborated through increased survival of sensory and motor neurons after SCI.
Conclusions
These results suggest intrathecal NAR as a promising candidate for medical therapeutics for SCI-induced sensory and motor dysfunction.
Keyword
Figure
Reference
-
1. Thuret S, Moon LD, Gage FH. 2006; Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 7:628–43. Erratum in: Nat Rev Neurosci 2006; 7: 902. DOI: 10.1038/nrn1955. PMID: 16858391.
Article2. Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. 2019; Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur J Pain. 23:750–64. DOI: 10.1002/ejp.1342. PMID: 30427581.
Article3. Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. 2018; Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res Bull. 143:217–24. DOI: 10.1016/j.brainresbull.2018.09.011. PMID: 30243665.
Article4. Naseem M, Parvez S. 2014; Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014:586270. DOI: 10.1155/2014/586270. PMID: 25587567. PMCID: PMC4283270.
Article5. Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. 2008; Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res. 45:149–56. DOI: 10.1111/j.1600-079X.2008.00569.x. PMID: 18298463.
Article6. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. 2012; Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 50:264–74. DOI: 10.1038/sc.2011.111. PMID: 21987065.
Article7. Faden AI, Wu J, Stoica BA, Loane DJ. 2016; Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 173:681–91. DOI: 10.1111/bph.13179. PMID: 25939377. PMCID: PMC4742301.
Article8. Franz S, Finnerup NB. Weidner N, Rupp R, Tansey K, editors. 2017. Diagnostics and treatment of pain in spinal cord injury. Neurological aspects of spinal cord injury. Springer;Cham: p. 283–302. https://doi.org/10.1007/978-3-319-46293-6_12. DOI: 10.1007/978-3-319-46293-6_12.
Article9. Backonja MM, Irving G, Argoff C. 2006; Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 10:34–8. DOI: 10.1007/s11916-006-0007-1. PMID: 16499828.
Article10. Hare JT, Elliott DP. 2003; Grapefruit juice and potential drug interactions. Consult Pharm. 18:466–72. PMID: 16563062.11. Palma-Duran SA, Caire-Juvera G, Robles-Burgeño Mdel R, Ortega-Vélez MI, Gutiérrez-Coronado Mde L, Almada Mdel C, et al. 2015; Serum levels of phytoestrogens as biomarkers of intake in Mexican women. Int J Food Sci Nutr. 66:819–25. DOI: 10.3109/09637486.2015.1092019. PMID: 26417700.
Article12. Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. 2016; Naringenin reduces inflammatory pain in mice. Neuropharmacology. 105:508–19. DOI: 10.1016/j.neuropharm.2016.02.019. PMID: 26907804.
Article13. Hu CY, Zhao YT. 2014; Analgesic effects of naringenin in rats with spinal nerve ligation-induced neuropathic pain. Biomed Rep. 2:569–73. DOI: 10.3892/br.2014.267. PMID: 24944810. PMCID: PMC4051469.
Article14. Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, et al. 2015; Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 37:924–33. DOI: 10.1179/1743132815Y.0000000079. PMID: 26187552.
Article15. Kaulaskar S, Bhutada P, Rahigude A, Jain D, Harle U. 2012; Effects of naringenin on allodynia and hyperalgesia in rats with chronic constriction injury-induced neuropathic pain. Zhong Xi Yi Jie He Xue Bao. 10:1482–9. DOI: 10.3736/jcim20121223. PMID: 23257145.
Article16. Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, Borghi SM, Bordignon J, Silva RL, et al. 2016; The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem. 33:8–14. DOI: 10.1016/j.jnutbio.2016.03.013. PMID: 27260463.
Article17. Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, et al. 2016; Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS One. 11:e0153015. DOI: 10.1371/journal.pone.0153015. PMID: 27045367. PMCID: PMC4821586.18. Fakhri S, Kiani A, Jalili C, Abbaszadeh F, Piri S, Farzaei MH, et al. 2021; Intrathecal administration of melatonin ameliorates the neuroinflammation- mediated sensory and motor dysfunction in a rat model of compression spinal cord injury. Curr Mol Pharmacol. 14:646–57. DOI: 10.2174/1874467213666201230101811. PMID: 33380311.
Article19. Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. 1994; A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 32:197–200. DOI: 10.1016/1056-8719(94)90087-6. PMID: 7881133.
Article20. Kauppila T. 2000; Cold exposure enhances tactile allodynia transiently in mononeuropathic rats. Exp Neurol. 161:740–4. DOI: 10.1006/exnr.1999.7287. PMID: 10686093.
Article21. Hama AT, Plum AW, Sagen J. 2010; Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol Biochem Behav. 97:249–55. DOI: 10.1016/j.pbb.2010.08.006. PMID: 20732348. PMCID: PMC2956774.
Article22. Janicki P, Libich J. 1979; Detection of antagonist activity for narcotic analgesics in mouse hot-plate test. Pharmacol Biochem Behav. 10:623–6. DOI: 10.1016/0091-3057(79)90244-2. PMID: 461487.
Article23. Basso DM, Beattie MS, Bresnahan JC. 1995; A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. DOI: 10.1089/neu.1995.12.1. PMID: 7783230.
Article24. Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. 2013; Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 110:238–44. DOI: 10.1016/j.pbb.2013.07.019. PMID: 23932920.
Article25. Rahman I, Kode A, Biswas SK. 2006; Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 1:3159–65. DOI: 10.1038/nprot.2006.378. PMID: 17406579.
Article26. Aebi H. Bergmeyer HU, editor. 1974. Catalase. Methods of enzymatic analysis. 2nd ed. Academic Press;New York: p. 673–84. https://doi.org/10.1016/B978-0-12-091302-2.50032-3 . DOI: 10.1016/B978-0-12-091302-2.50032-3.
Article27. Masoudi A, Dargahi L, Abbaszadeh F, Pourgholami MH, Asgari A, Manoochehri M, et al. 2017; Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav Brain Res. 329:104–10. DOI: 10.1016/j.bbr.2017.04.026. PMID: 28442361.
Article28. Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, et al. 2019; On the neuroprotective effects of naringenin: pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules. 9:690. DOI: 10.3390/biom9110690. PMID: 31684142. PMCID: PMC6920995.
Article29. Mao L, Wang H, Qiao L, Wang X. 2010; Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010:238321. DOI: 10.1155/2010/238321. PMID: 20862369. PMCID: PMC2938451.30. Alghamdi BS. 2018; The neuroprotective role of melatonin in neurological disorders. J Neurosci Res. 96:1136–49. DOI: 10.1002/jnr.24220. PMID: 29498103. PMCID: PMC6001545.
Article31. Stetler-Stevenson WG. 1996; Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 148:1345–50. PMID: 8623905. PMCID: PMC1861572.32. Shibata N, Ohnuma T, Higashi S, Usui C, Ohkubo T, Kitajima A, et al. 2005; Genetic association between matrix metalloproteinase MMP-9 and MMP-3 polymorphisms and Japanese sporadic Alzheimer's disease. Neurobiol Aging. 26:1011–4. DOI: 10.1016/j.neurobiolaging.2004.09.004. PMID: 15748780.
Article33. Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. 2002; Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp Neurol. 178:13–20. DOI: 10.1006/exnr.2002.8019. PMID: 12460604.
Article34. Beuche W, Yushchenko M, Mäder M, Maliszewska M, Felgenhauer K, Weber F. 2000; Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport. 11:3419–22. DOI: 10.1097/00001756-200011090-00003. PMID: 11095490.
Article35. Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. 2002; Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 22:7526–35. DOI: 10.1523/JNEUROSCI.22-17-07526.2002. PMID: 12196576. PMCID: PMC2792199.
Article36. Yang R, Guo L, Wang P, Huang L, Tang Y, Wang W, et al. 2014; Epidemiology of spinal cord injuries and risk factors for complete injuries in Guangdong, China: a retrospective study. PLoS One. 9:e84733. DOI: 10.1371/journal.pone.0084733. PMID: 24489652. PMCID: PMC3904832. PMID: 9bcaf3aad5fb45dea562cdd91b88f449.
Article37. Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, et al. 2003; An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 23:10107–15. DOI: 10.1523/JNEUROSCI.23-31-10107.2003. PMID: 14602826. PMCID: PMC6740867.
Article38. Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. 2011; Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 8:206–20. DOI: 10.1007/s13311-011-0038-0. PMID: 21455784. PMCID: PMC3077748.
Article39. Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L, et al. 2014; Melatonin improves functional outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal cord injury in rats. Acta Neurochir (Wien). 156:2173–82. DOI: 10.1007/s00701-014-2119-4. PMID: 24879621.
Article40. Jang JW, Lee JK, Kim SH. 2011; Activation of matrix metalloproteinases-9 after photothrombotic spinal cord injury model in rats. J Korean Neurosurg Soc. 50:288–92. DOI: 10.3340/jkns.2011.50.4.288. PMID: 22200008. PMCID: PMC3243829.
Article41. Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, et al. 2014; Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res. 39:1405–15. DOI: 10.1007/s11064-014-1326-y. PMID: 24842554.
Article42. Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. 2006; Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 26:9841–50. DOI: 10.1523/JNEUROSCI.1993-06.2006. PMID: 17005848. PMCID: PMC2659718.
Article43. Shi LB, Tang PF, Zhang W, Zhao YP, Zhang LC, Zhang H. 2016; Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene. 592:128–33. DOI: 10.1016/j.gene.2016.07.037. PMID: 27432064.
Article44. Puspitasari V, Gunawan PY, Wiradarma HD, Hartoyo V. 2019; Glial fibrillary acidic protein serum level as a predictor of clinical outcome in ischemic stroke. Open Access Maced J Med Sci. 7:1471–4. DOI: 10.3889/oamjms.2019.326. PMID: 31198457. PMCID: PMC6542382.
Article45. Khajevand-Khazaei MR, Ziaee P, Motevalizadeh SA, Rohani M, Afshin-Majd S, Baluchnejadmojarad T, et al. 2018; Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur J Pharmacol. 826:114–22. DOI: 10.1016/j.ejphar.2018.03.001. PMID: 29518393.
Article46. Krishna Chandran AM, Christina H, Das S, Mumbrekar KD, Satish Rao BS. 2019; Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ Toxicol Pharmacol. 71:103224. DOI: 10.1016/j.etap.2019.103224. PMID: 31376681.
Article47. Chtourou Y, Fetoui H, Gdoura R. 2014; Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res. 158:376–83. DOI: 10.1007/s12011-014-9948-0. PMID: 24682942.
Article48. Muthaiah VP, Venkitasamy L, Michael FM, Chandrasekar K, Venkatachalam S. 2013; Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. J Pharmacol Pharmacother. 4:192–7. DOI: 10.4103/0976-500X.114599. PMID: 23960424. PMCID: PMC3746302.
Article49. Xu XH, Ma CM, Han YZ, Li Y, Liu C, Duan ZH, et al. 2015; Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch Biol Sci. 67:639–46. https://doi.org/10.2298/ABS140811023X. DOI: 10.2298/ABS140811023X.
Article50. Giangregorio L, McCartney N. 2006; Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 29:489–500. DOI: 10.1080/10790268.2006.11753898. PMID: 17274487. PMCID: PMC1949032.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities
- Effect of Intrathecal Ginsenosides on Mechanical Allodynia in a Neuropathic Rat Model
- Synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model
- The Effect of Intrathecal Gabapentin on Mechanical and Thermal Hyperalgesia in Neuropathic Rats Induced by Spinal Nerve Ligation
- The Mechanical Antiallodynic Effect of Intrathecal Lamotrigine in Rats with Spinal Nerve Ligation