Korean J Pain.
2022 Jul;35(3):271-279. 10.3344/kjp.2022.35.3.271.
Synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model
- Affiliations
-
- 1Neuroscience and Inflammation Unit, Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria
- 2Neuroscience and Inflammation Unit, Department of Physiology, Adeleke University, Ede, Osun State, Nigeria
- 3Department of Nursing, Faculty of Social Welfare and Health Sciences, University of Haifa, Haifa, Israel
- KMID: 2530982
- DOI: http://doi.org/10.3344/kjp.2022.35.3.271
Abstract
- Background
Inflammation is known to underlie the pathogenesis in neuropathic pain. This study investigated the anti-inflammatory and neuroprotective mechanisms involved in antinociceptive effects of co-administration of acetaminophen and L-carnosine in chronic constriction injury (CCI)-induced peripheral neuropathy in male Wistar rats.
Methods
Fifty-six male Wistar rats were randomly divided into seven experimental groups (n = 8) treated with normal saline/acetaminophen/acetaminophen + L-carnosine. CCI was used to induce neuropathic pain in rats. Hyperalgesia and allodynia were assessed using hotplate and von Frey tests, respectively. Investigation of spinal proinflammatory cytokines and antioxidant system were carried out after twenty-one days of treatment.
Results
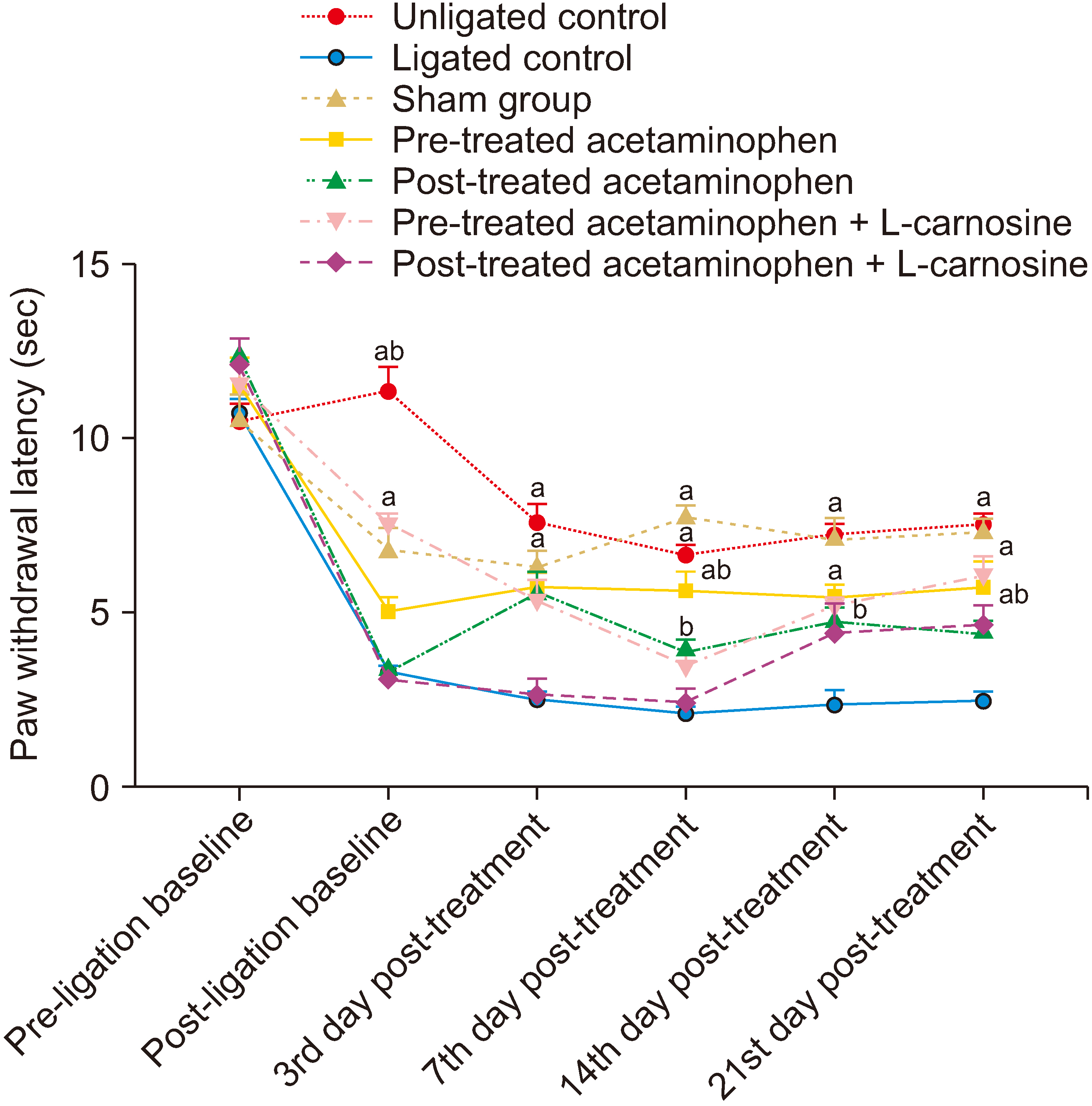
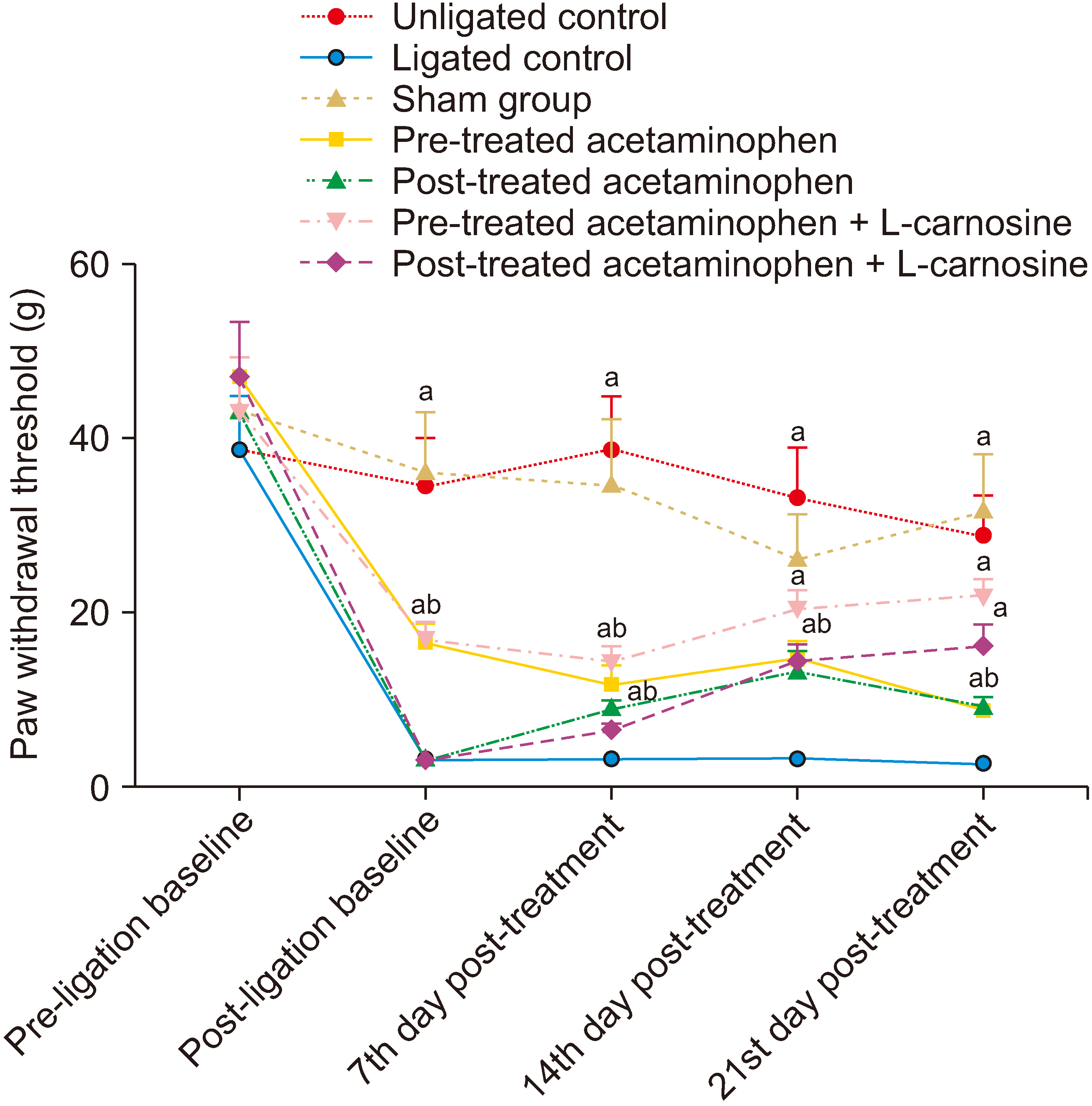
The results showed that the co-administration of acetaminophen and Lcarnosine significantly (P < 0.001) increased the paw withdrawal threshold to thermal and mechanical stimuli in ligated rats compared to the ligated naïve group. There was a significant (P < 0.001) decrease in the levels of nuclear factor kappa light chain enhancer B cell inhibitor, calcium ion, interleukin-1-beta, and tumour necrotic factor-alpha in the spinal cord of the group coadministered with acetaminophen and L-carnosine compared to the ligated control group. Co-administration with acetaminophen and L-carnosine increased the antioxidant enzymatic activities and reduced the lipid peroxidation in the spinal cord.
Conclusions
Co-administration of acetaminophen and L-carnosine has anti-inflammatory effects as a mechanism that mediate its antinociceptive effects in CCIinduced peripheral neuropathy in Wistar rat.
Keyword
Figure
Reference
-
1. Murphy D, Lester D, Clay Smither F, Balakhanlou E. 2020; Peripheral neuropathic pain. NeuroRehabilitation. 47:265–83. DOI: 10.3233/NRE-208002. PMID: 32986619.
Article2. Bravo L, Llorca-Torralba M, Suárez-Pereira I, Berrocoso E. 2020; Pain in neuropsychiatry: insights from animal models. Neurosci Biobehav Rev. 115:96–115. DOI: 10.1016/j.neubiorev.2020.04.029. PMID: 32437745.
Article3. Finnerup NB, Kuner R, Jensen TS. 2021; Neuropathic pain: from mechanisms to treatment. Physiol Rev. 101:259–301. DOI: 10.1152/physrev.00045.2019. PMID: 32584191.
Article4. Peng J, Gu N, Zhou L, Eyo UB, Murugan M, Gan WB, et al. 2016; Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 7:12029. DOI: 10.1038/ncomms12029. PMID: 27349690. PMCID: PMC4931235. PMID: 718c07a2fe284ea1b1b6c6e085269f46.
Article5. Fonseca MM, Davoli-Ferreira M, Santa-Cecília F, Guimarães RM, Oliveira FFB, Kusuda R, et al. 2020; IL-27 counteracts neuropathic pain development through induction of IL-10. Front Immunol. 10:3059. DOI: 10.3389/fimmu.2019.03059. PMID: 32047492. PMCID: PMC6997342. PMID: 9d29671462ae4393ba0ae6f5d8f3cd14.
Article6. O'Reilly ML, Tom VJ. 2020; Neuroimmune system as a driving force for plasticity following CNS injury. Front Cell Neurosci. 14:187. DOI: 10.3389/fncel.2020.00187. PMID: 32792908. PMCID: PMC7390932. PMID: 88a6f725a1144e30817ab4ac7eb2fddf.7. Tang J, Bair M, Descalzi G. 2021; Reactive astrocytes: critical players in the development of chronic pain. Front Psychiatry. 12:682056. DOI: 10.3389/fpsyt.2021.682056. PMID: 34122194. PMCID: PMC8192827. PMID: 8bba54cfab8244f8b420e002cfecfbaa.
Article8. Salvemini D, Little JW, Doyle T, Neumann WL. 2011; Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 51:951–66. DOI: 10.1016/j.freeradbiomed.2011.01.026. PMID: 21277369. PMCID: PMC3134634.
Article9. Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C. 2013; Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain. 154:677–89. DOI: 10.1016/j.pain.2012.12.025. PMID: 23433945.
Article10. Roy S, Simalti AK. 2018; Comparison of antipyretic efficacy of intravenous (IV) acetaminophen versus oral (PO) acetaminophen in the management of fever in children. Indian J Pediatr. 85:1–4. DOI: 10.1007/s12098-017-2457-3. PMID: 28887752.
Article11. Trexler ET, Smith-Ryan AE, Stout JR, Hoffman JR, Wilborn CD, Sale C, et al. 2015; International society of sports nutrition position stand: beta-alanine. J Int Soc Sports Nutr. 12:30. DOI: 10.1186/s12970-015-0090-y. PMID: 26175657. PMCID: PMC4501114.
Article12. Baguet A, Koppo K, Pottier A, Derave W. 2010; Beta-alanine supplementation reduces acidosis but not oxygen uptake response during high-intensity cycling exercise. Eur J Appl Physiol. 108:495–503. DOI: 10.1007/s00421-009-1225-0. PMID: 19841932.
Article13. Boldyrev AA, Aldini G, Derave W. 2013; Physiology and pathophysiology of carnosine. Physiol Rev. 93:1803–45. DOI: 10.1152/physrev.00039.2012. PMID: 24137022.
Article14. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. 2020; The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 40:1769–77. DOI: 10.1177/0271678X20943823. PMID: 32663096. PMCID: PMC7430098.
Article15. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI: 10.1016/0304-3959(88)90209-6. PMID: 2837713.
Article16. Aydın AF, Küçükgergin C, Ozdemirler-Erata G, Koçak-Toker N, Uysal M. 2010; The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology. 11:103–9. DOI: 10.1007/s10522-009-9232-4. PMID: 19430956.
Article17. Im KS, Jung HJ, Kim JB, Lee JM, Park HJ, Joo CH, et al. 2012; The antinociceptive effect of acetaminophen in a rat model of neuropathic pain. Kaohsiung J Med Sci. 28:251–8. DOI: 10.1016/j.kjms.2011.11.003. PMID: 22531303.
Article18. Bakare AO, Owoyele BV. 2020; Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J Pain. 33:13–22. DOI: 10.3344/kjp.2020.33.1.13. PMID: 31888313. PMCID: PMC6944371.
Article19. Misra HP, Fridovich I. 1972; The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 247:3170–5. DOI: 10.1016/S0021-9258(19)45228-9. PMID: 4623845.
Article20. Ellman GL. 1959; Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–7. DOI: 10.1016/0003-9861(59)90090-6. PMID: 13650640.
Article21. Gutteridge JM, Wilkins S. 1982; Copper-dependent hydroxyl radical damage to ascorbic acid: formation of a thiobarbituric acid-reactive product. FEBS Lett. 137:327–30. DOI: 10.1016/0014-5793(82)80377-3. PMID: 6277694.22. Muthuraman A, Diwan V, Jaggi AS, Singh N, Singh D. 2008; Ameliorative effects of Ocimum sanctum in sciatic nerve transection-induced neuropathy in rats. J Ethnopharmacol. 120:56–62. DOI: 10.1016/j.jep.2008.07.049. PMID: 18762236.
Article23. Kim SH, Nam JS, Choi DK, Koh WW, Suh JH, Song JG, et al. 2011; Tumor necrosis factor-alpha and apoptosis following spinal nerve ligation injury in rats. Korean J Pain. 24:185–90. DOI: 10.3344/kjp.2011.24.4.185. PMID: 22220239. PMCID: PMC3248581.
Article24. Shim DJ, Yang L, Reed JG, Noebels JL, Chiao PJ, Zheng H. 2011; Disruption of the NF-κB/IκBα autoinhibitory loop improves cognitive performance and promotes hyperexcitability of hippocampal neurons. Mol Neurodegener. 6:42. DOI: 10.1186/1750-1326-6-42. PMID: 21663635. PMCID: PMC3141554.
Article25. Dresselhaus EC, Meffert MK. 2019; Cellular specificity of NF-κB function in the nervous system. Front Immunol. 10:1043. DOI: 10.3389/fimmu.2019.01043. PMID: 31143184. PMCID: PMC6520659.
Article26. Bakare AO, Owoyele BV. 2021; Bromelain reduced pro-inflammatory mediators as a common pathway that mediate antinociceptive and anti-anxiety effects in sciatic nerve ligated Wistar rats. Sci Rep. 11:289. DOI: 10.1038/s41598-020-79421-9. PMID: 33432004. PMCID: PMC7801445. PMID: d37c0ad8795f4313b44249b638a506ae.
Article27. Rajanandh MG, Kosey S, Prathiksha G. 2014; Assessment of antioxidant supplementation on the neuropathic pain score and quality of life in diabetic neuropathy patients - a randomized controlled study. Pharmacol Rep. 66:44–8. DOI: 10.1016/j.pharep.2013.08.003. PMID: 24905305.
Article28. Zhou YQ, Liu DQ, Chen SP, Chen N, Sun J, Wang XM, et al. 2020; Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol Sin. 41:1041–8. DOI: 10.1038/s41401-020-0394-6. PMID: 32203087. PMCID: PMC7470811.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Sec-O-glucosylhamaudol mitigates inflammatory processes and autophagy via p38/JNK MAPK signaling in a rat neuropathic pain model
- Aurantio-obtusin exerts an anti-inflammatory effect on acute kidney injury by inhibiting NF-κκB pathway