Korean J Physiol Pharmacol.
2022 Jul;26(4):277-285. 10.4196/kjpp.2022.26.4.277.
Inhibitory effects of the atypical antipsychotic, clozapine, on voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells
- Affiliations
-
- 1Institute of Medical Sciences, Department of Physiology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 2Department of Medical Environmental Biology and Tropical Medicine, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 3Department of Pharmacology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 4Department of Molecular and Cellular Biochemistry, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 5Institute of Medical Sciences, Department of Urology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 6Department of Biomedical Engineering and Center for Marine-Integrated Biomedical Technology (BK21 Plus), Pukyong National University, Busan 48513, Korea
- 7Department of Microbiology, College of Medicine, Inje University, Busan 48516, Korea
- KMID: 2530947
- DOI: http://doi.org/10.4196/kjpp.2022.26.4.277
Abstract
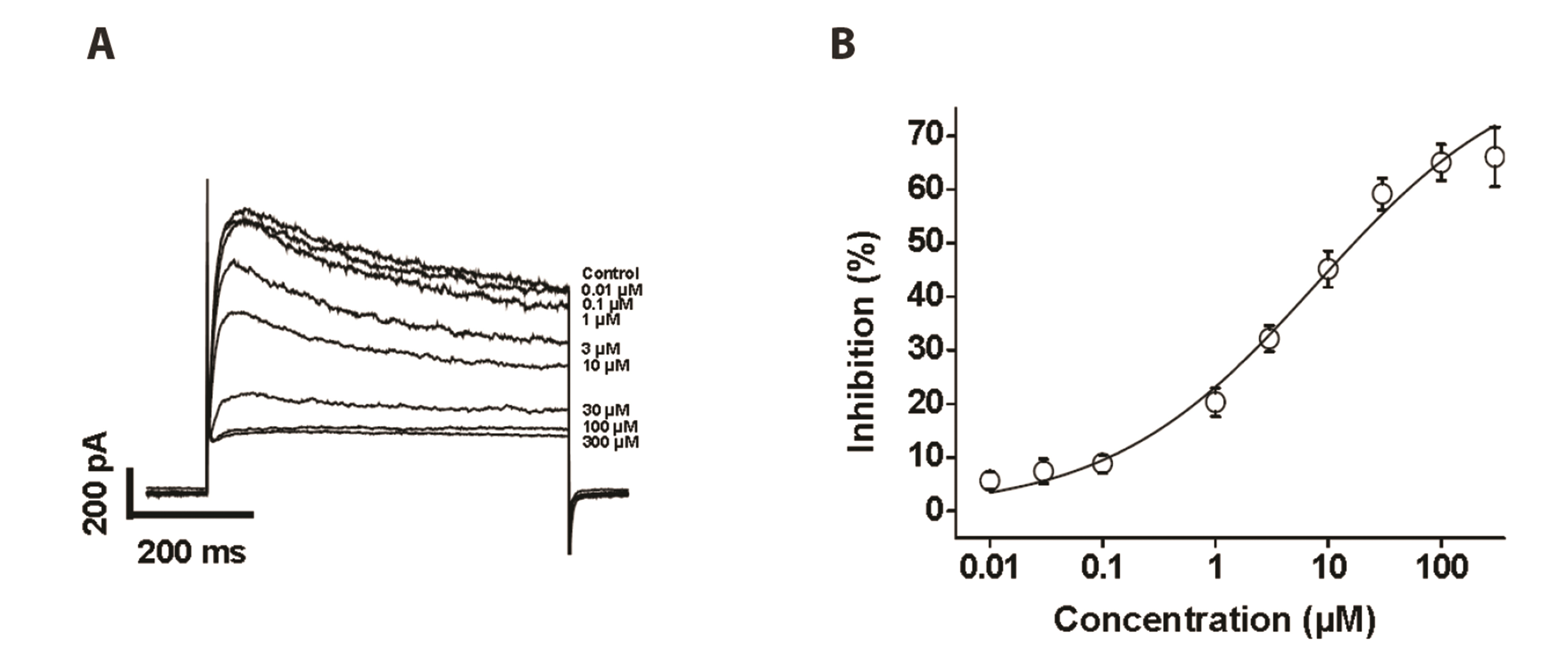
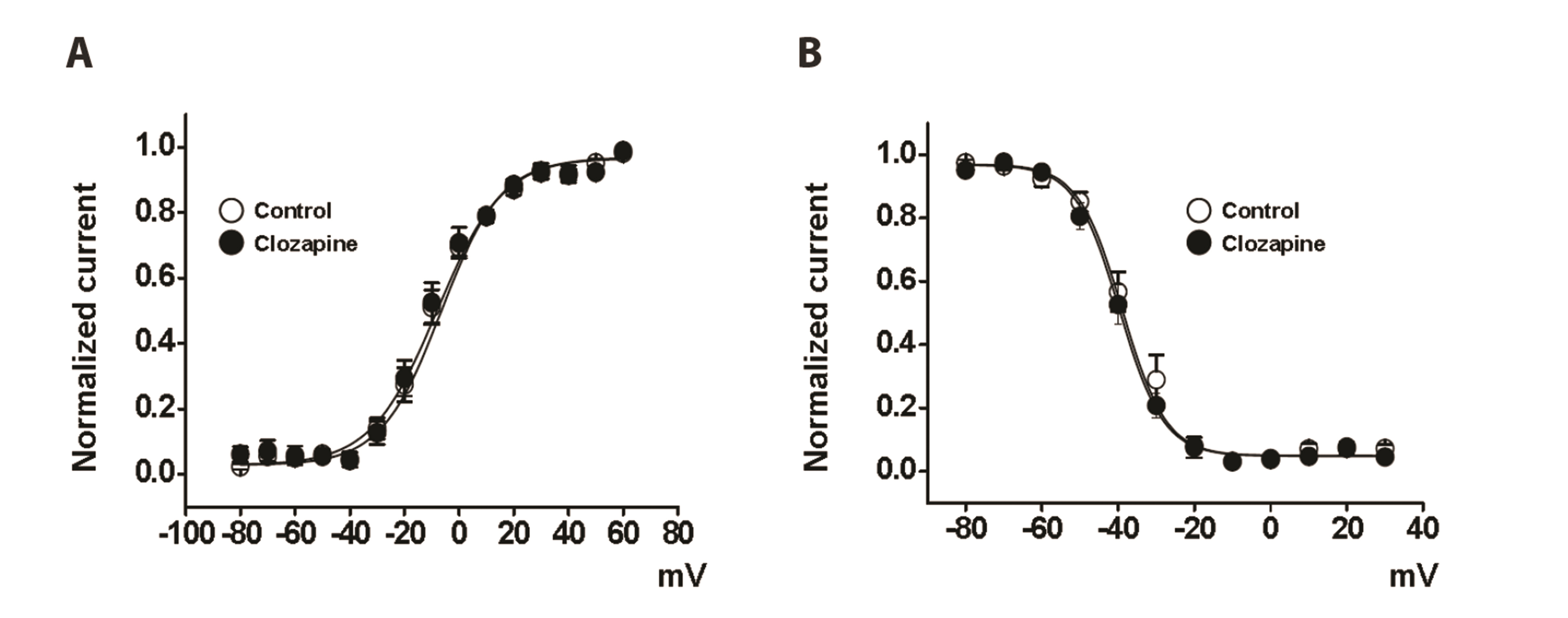
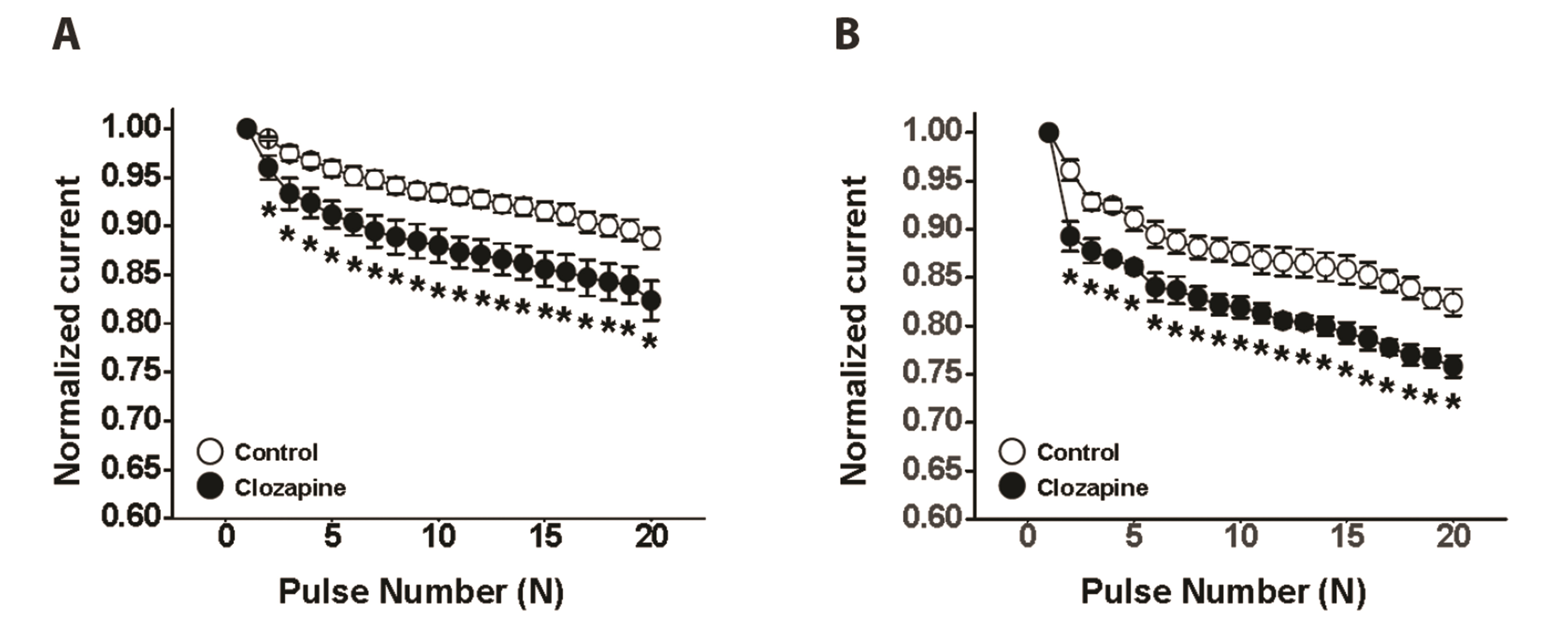
- To investigate the adverse effects of clozapine on cardiovascular ion channels, we examined the inhibitory effect of clozapine on voltage-dependent K+(Kv) channels in rabbit coronary arterial smooth muscle cells. Clozapine-induced inhibition of Kv channels occurred in a concentration-dependent manner with an halfinhibitory concentration value of 7.84 ± 4.86 µM and a Hill coefficient of 0.47 ± 0.06. Clozapine did not shift the steady-state activation or inactivation curves, suggesting that it inhibited Kv channels regardless of gating properties. Application of train pulses (1 and 2 Hz) progressively augmented the clozapine-induced inhibition of Kv channels in the presence of the drug. Furthermore, the recovery time constant from inactivation was increased in the presence of clozapine, suggesting that clozapineinduced inhibition of Kv channels is use (state)-dependent. Pretreatment of a Kv1.5 subtype inhibitor decreased the Kv current amplitudes, but additional application of clozapine did not further inhibit the Kv current. Pretreatment with Kv2.1 or Kv7 subtype inhibitors partially blocked the inhibitory effect of clozapine. Based on these results, we conclude that clozapine inhibits arterial Kv channels in a concentrationand use (state)-dependent manner. Kv1.5 is the major subtype involved in clozapineinduced inhibition of Kv channels, and Kv2.1 and Kv7 subtypes are partially involved.
Figure
Cited by 1 articles
-
Haloperidol, a typical antipsychotic, inhibits 5-HT3 receptor-mediated currents in NCB-20 cells: a whole-cell patch-clamp study
Yong Soo Park, Gyu Min Kim, Ho Jun Sung, Ju Yeong Yu, Ki-Wug Sung
Korean J Physiol Pharmacol. 2025;29(3):349-358. doi: 10.4196/kjpp.24.320.
Reference
-
1. Meltzer HY, Massey BW. 2011; The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 11:59–67. DOI: 10.1016/j.coph.2011.02.007. PMID: 21420906. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79953689886&origin=inward.
Article2. Miyamoto S, Duncan GE, Marx CE, Lieberman JA. 2005; Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 10:79–104. DOI: 10.1038/sj.mp.4001556. PMID: 15289815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=12344326514&origin=inward.
Article3. Khokhar JY, Henricks AM, Sullivan EDK, Green AI. 2018; Unique effects of clozapine: a pharmacological perspective. Adv Pharmacol. 82:137–162. DOI: 10.1016/bs.apha.2017.09.009. PMID: 29413518. PMCID: PMC7197512. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040459202&origin=inward.
Article4. Krupp P, Barnes P. 2005; Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. (17):38–40. DOI: 10.1192/S0007125000296906. PMID: 15289815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0026559173&origin=inward.
Article5. Young CR, Bowers MB Jr, Mazure CM. 1998; Management of the adverse effects of clozapine. Schizophr Bull. 24:381–390. DOI: 10.1093/oxfordjournals.schbul.a033333. PMID: 9718630. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031849782&origin=inward.
Article6. Choi KH, Rhim H. 2010; Inhibition of recombinant Ca(v)3.1 (alpha(1G)) T-type calcium channels by the antipsychotic drug clozapine. Eur J Pharmacol. 626:123–130. DOI: 10.1016/j.ejphar.2009.09.035. PMID: 19782679. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70649101232&origin=inward.
Article7. Lee SY, Kim YJ, Kim KT, Choe H, Jo SH. 2006; Blockade of HERG human K+ channels and IKr of guinea-pig cardiomyocytes by the antipsychotic drug clozapine. Br J Pharmacol. 148:499–509. DOI: 10.1038/sj.bjp.0706744. PMID: 16633353. PMCID: PMC1751795. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33745094425&origin=inward.
Article8. Leung JY, Barr AM, Procyshyn RM, Honer WG, Pang CC. 2012; Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system. Pharmacol Ther. 135:113–122. DOI: 10.1016/j.pharmthera.2012.04.003. PMID: 22565090. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84862687967&origin=inward.
Article9. Howell S, Yarovova E, Khwanda A, Rosen SD. 2019; Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart. 105:1852–1859. DOI: 10.1136/heartjnl-2017-312107. PMID: 31439658. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071634649&origin=inward.
Article10. Nelson MT, Quayle JM. 1995; Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 268(4 Pt 1):C799–C822. DOI: 10.1152/ajpcell.1995.268.4.C799. PMID: 7733230. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029108913&origin=inward.
Article11. Keynes RD. 1975; The ionic channels in excitable membranes. Ciba Found Symp. (31):191–203. DOI: 10.1002/9780470720134.ch11. PMID: 1041242.
Article12. Kim HS, Li H, Kim HW, Shin SE, Seo MS, An JR, Ha KS, Han ET, Hong SH, Choi IW, Choi G, Lee DS, Park WS. 2017; Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Korean J Physiol Pharmacol. 21:415–421. DOI: 10.4196/kjpp.2017.21.4.415. PMID: 28706455. PMCID: PMC5507780. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85023742682&origin=inward.
Article13. Cook NS. 1989; Effect of some potassium channel blockers on contractile responses of the rabbit aorta. J Cardiovasc Pharmacol. 13:299–306. DOI: 10.1097/00005344-198902000-00019. PMID: 2468961. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0024520186&origin=inward.
Article14. Sobey CG. 2001; Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 21:28–38. DOI: 10.1161/01.ATV.21.1.28. PMID: 11145930. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035162575&origin=inward.
Article15. Nieves-Cintrón M, Syed AU, Nystoriak MA, Navedo MF. 2018; Regulation of voltage-gated potassium channels in vascular smooth muscle during hypertension and metabolic disorders. Microcirculation. 25:e12423. DOI: 10.1111/micc.12423. PMID: 29044853. PMCID: PMC5760350. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040240767&origin=inward.
Article16. Park WS, Ko JH, Kim N, Son YK, Kang SH, Warda M, Jung ID, Park YM, Han J. 2007; Increased inhibition of inward rectifier K+ channels by angiotensin II in small-diameter coronary artery of isoproterenol-induced hypertrophied model. Arterioscler Thromb Vasc Biol. 27:1768–1775. DOI: 10.1161/ATVBAHA.107.143339. PMID: 17525364. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34547626373&origin=inward.
Article17. Jackson WF. 2018; KV channels and the regulation of vascular smooth muscle tone. Microcirculation. 25:e12421. DOI: 10.1111/micc.12421. PMID: 28985443. PMCID: PMC5760307. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040242982&origin=inward.18. Kang M, An JR, Seo MS, Jung HS, Heo R, Park H, Song G, Jung WK, Choi IW, Park WS. 2021; Atypical antipsychotic olanzapine inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Pharmacol Rep. 73:1724–1733. DOI: 10.1007/s43440-021-00299-z. PMID: 34146337. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85108274680&origin=inward.
Article19. An JR, Seo MS, Jung HS, Heo R, Kang M, Ha KS, Park H, Park WS. 2020; The inhibitory effect of ziprasidone on voltage-dependent K+ channels in coronary arterial smooth muscle cells. Biochem Biophys Res Commun. 529:191–197. DOI: 10.1016/j.bbrc.2020.06.038. PMID: 32703410. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087063071&origin=inward.
Article20. An JR, Seo MS, Jung HS, Kang M, Heo R, Bae YM, Han ET, Yang SR, Park WS. 2020; Inhibition of voltage-dependent K+ channels by iloperidone in coronary arterial smooth muscle cells. J Appl Toxicol. 40:1297–1305. DOI: 10.1002/jat.3986. PMID: 32285496. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85083291351&origin=inward.
Article21. An JR, Seo MS, Jung HS, Li H, Jung WK, Choi IW, Ha KS, Han ET, Hong SH, Park H, Bae YM, Park WS. 2020; Inhibition by the atypical antipsychotic risperidone of voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Eur J Pharmacol. 874:173027. DOI: 10.1016/j.ejphar.2020.173027. PMID: 32084421. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079842287&origin=inward.22. Dogan MF, Yildiz O, Arslan SO, Ulusoy KG. 2019; Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol. 33:504–523. DOI: 10.1111/fcp.12461. PMID: 30851197. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85063670988&origin=inward.
Article23. Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. 2001; Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 89:1030–1037. DOI: 10.1161/hh2301.100817. PMID: 11717160. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035941423&origin=inward.
Article24. Li Q, Zhang R, Lü CL, Liu Y, Wang Z, Zhu DL. 2006; [The role of subtypes of voltage-gated K+ channels in pulmonary vasoconstriction induced by 15-hydroeicosatetraenoic acid]. Yao Xue Xue Bao. 41:412–417. Chinese. PMID: 16848316. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33745784809&origin=inward.25. Morales-Cano D, Moreno L, Barreira B, Pandolfi R, Chamorro V, Jimenez R, Villamor E, Duarte J, Perez-Vizcaino F, Cogolludo A. 2015; Kv7 channels critically determine coronary artery reactivity: left-right differences and down-regulation by hyperglycaemia. Cardiovasc Res. 106:98–108. DOI: 10.1093/cvr/cvv020. PMID: 25616413. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84926616152&origin=inward.
Article26. An JR, Li H, Seo MS, Park WS. 2018; Inhibition of voltage-dependent K+ current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone. Korean J Physiol Pharmacol. 22:597–605. DOI: 10.4196/kjpp.2018.22.5.597. PMID: 30181706. PMCID: PMC6115351. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052872084&origin=inward.
Article27. Lagrutta A, Wang J, Fermini B, Salata JJ. 2006; Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 317:1054–1063. DOI: 10.1124/jpet.106.101162. PMID: 16522807. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33646766140&origin=inward.
Article28. Herrington J. 2007; Gating modifier peptides as probes of pancreatic beta-cell physiology. Toxicon. 49:231–238. DOI: 10.1016/j.toxicon.2006.09.012. PMID: 17101164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846413179&origin=inward.
Article29. Schnee ME, Brown BS. 1998; Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther. 286:709–717. PMID: 9694925. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032442105&origin=inward.30. Fitton A, Heel RC. 1990; Clozapine. A review of its pharmacological properties, and therapeutic use in schizophrenia. Drugs. 40:722–747. DOI: 10.2165/00003495-199040050-00007. PMID: 2292234. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025599851&origin=inward.31. Tassaneeyakul W, Kittiwattanagul K, Vannaprasaht S, Kampan J, Tawalee A, Puapairoj P, Tiamkao S, Juthagridsada S, Kukongviriyapan V, Tassaneeyakul W. 2005; Steady-state bioequivalence study of clozapine tablet in schizophrenic patients. J Pharm Pharm Sci. 8:47–53. PMID: 15946597. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=27444437220&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- The Effect of Midazolam on Outward K+ Channel Currents in Rabbit Cerebral Arterial Smooth Muscle Cells
- Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent Kâ» channels in coronary arterial smooth muscle cells
- Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells
- The alteration of Ca2+-activated K+ channels in coronary arterial smooth muscle cells isolated from isoproterenol-induced cardiac hypertrophy in rabbit