Blood Res.
2022 Jun;57(2):144-151. 10.5045/br.2022.2021137.
Safety and efficacy of nilotinib in adult patients with chronic myeloid leukemia: a post-marketing surveillance study in Korea
- Affiliations
-
- 1Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 2Department of Hematology-Oncology, School of Medicine, Kyungpook National University, Daegu, Korea
- 3Department of Internal Medicine, 3 University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 5Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea
- 7Division of Hematology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea
- 8Division of Hematology, Department of Internal Medicine, College of Medicine, Chungnam National Univeristy, Daejeon, Korea
- 9Division of Hematology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 10Department of Hematology and Oncology, Ewha Womans University College of Medicine, Seoul, Korea
- 11Division of Hematology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 12Department of Internal Medicine, Inje University, Sanggye-Paik Hospital, Seoul, Korea
- 13Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- 14Division of Hematology-Oncology, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea
- 15Department of Hematology-Oncology, Wonkwang University School of Medicine, Iksan, Korea
- 16Division of Hematology-Oncology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 17Department of Internal Medicine, Gyeongsang Institute of Health Sciences, Gyeongsang National University College of Medicine and Gyeongsang National University Changwon Hospital, Changwon, Korea
- 18Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 19Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea
- 20Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 21Division of Oncology-Hematology, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 22Division of Oncology-Hematology, Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- 23Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea
- 24Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea
- 25Department of Internal Medicine, Inje University College of Medicine, Haeundae Paik Hospital, Busan, Korea
- 26Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 27Novartis Korea Ltd., Seoul, Korea
- 28Department of Hematology, Uijeongbu Eulji Medical Center, Leukemia Omics Research Institute, Eulji University Uijeongbu Campus, Uijeongbu, Korea
- KMID: 2530879
- DOI: http://doi.org/10.5045/br.2022.2021137
Abstract
- Background
Nilotinib is a tyrosine kinase inhibitor approved by the Ministry of Food and Drug Safety for frontline and 2nd line treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML). This study aimed to confirm the safety and efficacy of nilotinib in routine clinical practice within South Korea.
Methods
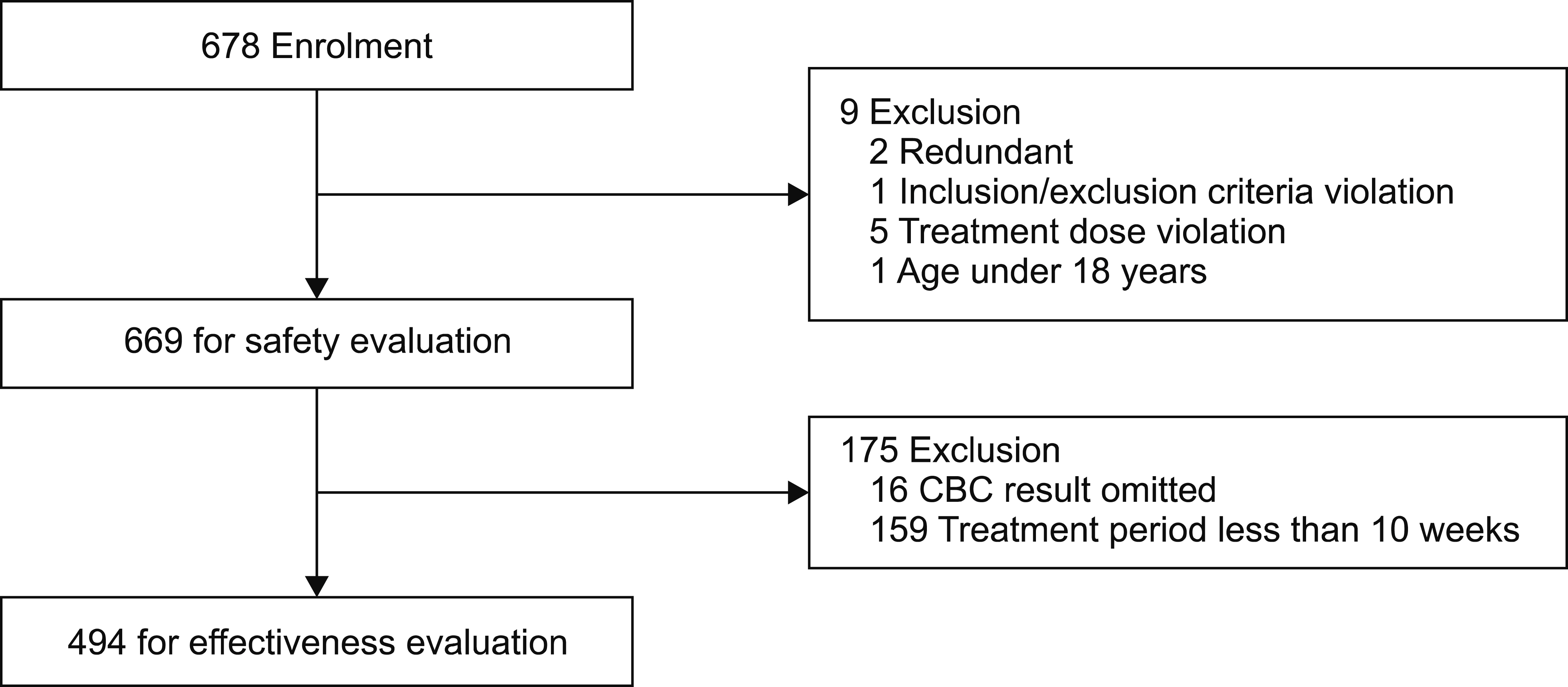
An open-label, multicenter, single-arm, 12-week observational post-marketing surveillance (PMS) study was conducted on 669 Korean adult patients with Ph + CML from December 24, 2010, to December 23, 2016. The patients received nilotinib treatment in routine clinical practice settings. Safety was evaluated by all types of adverse events (AEs) during the study period, and efficacy was evaluated by the complete hematological response (CHR) and cytogenetic response.
Results
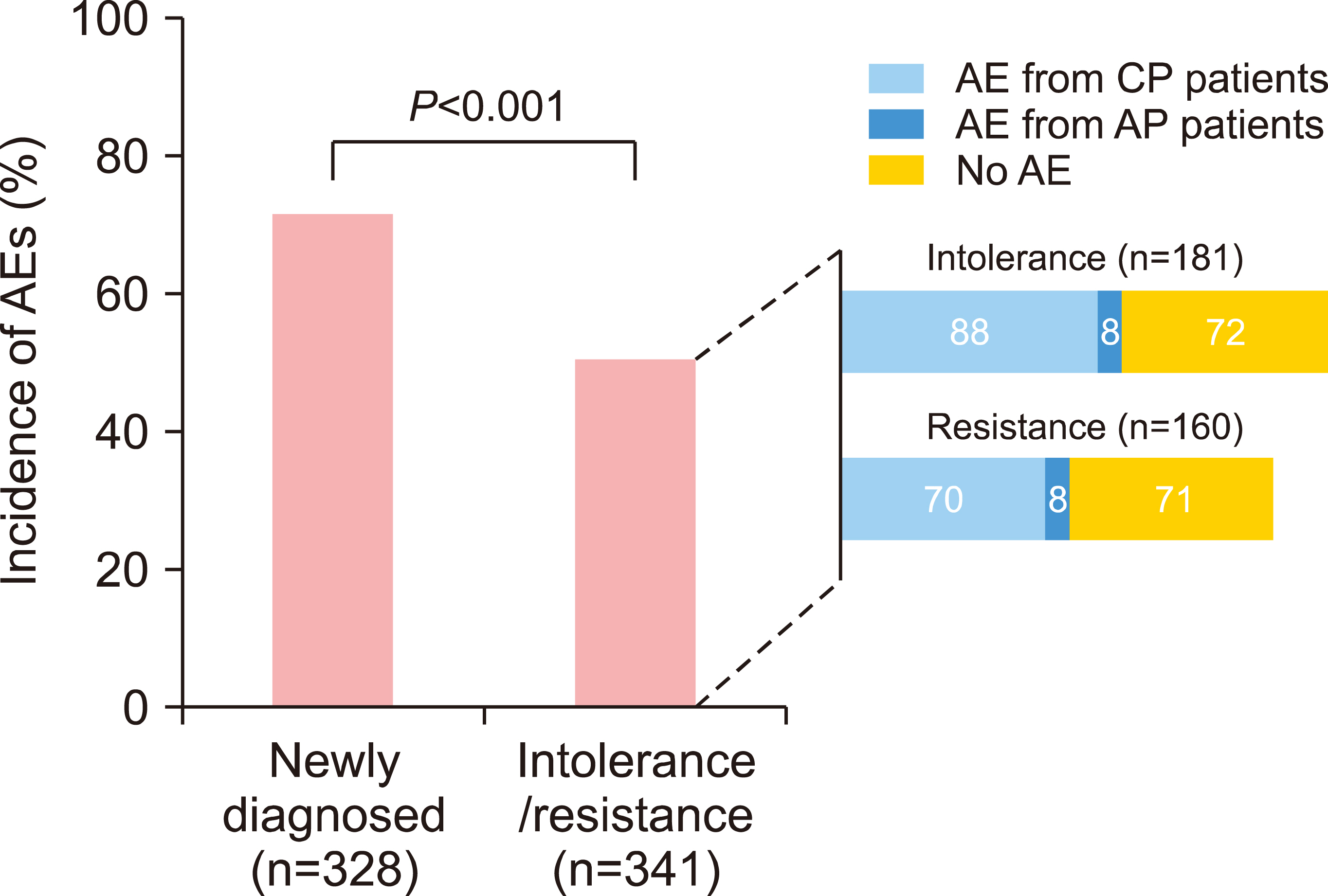
During the study period, AEs occurred in 61.3% (410 patients, 973 events), adverse drug reactions (ADRs) in 40.5% (271/669 patients, 559 events), serious AEs in 4.5% (30 patients, 37 events), and serious ADRs in 0.7% (5 patients, 8 events). Furthermore, unexpected AEs occurred at a rate of 6.9% (46 patients, 55 events) and unexpected ADRs at 1.2% (8 patients, 8 events). As for the efficacy results, CHR was achieved in 89.5% (442/494 patients), and minor cytogenetic response or major cytogenetic response was achieved in 85.8% (139/162 patients).
Conclusion
This PMS study shows consistent results in terms of safety and efficacy compared with previous studies. Nilotinib was well tolerated and efficacious in adult Korean patients with Ph + CML in routine clinical practice settings.
Keyword
Figure
Reference
-
1. Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. 1999; The biology of chronic myeloid leukemia. N Engl J Med. 341:164–72. DOI: 10.1056/NEJM199907153410306. PMID: 10403855.
Article2. Sawyers CL. 1999; Chronic myeloid leukemia. N Engl J Med. 340:1330–40. DOI: 10.1056/NEJM199904293401706. PMID: 10219069.
Article3. Jabbour E, Hochhaus A, le Coutre P, et al. 2008; Minimal cross- intolerance between nilotinib and imatinib in patients with imatinib-intolerant chronic myelogenous leukemia (CML) in chronic phase (CP) or accelerated phase (AP). J Clin Oncol (ASCO Annual Meeting Abstracts). 26(Suppl):7063. DOI: 10.1200/jco.2008.26.15_suppl.7063.
Article4. Saglio G, Kim DW, Issaragrisil S, et al. 2010; Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 362:2251–9. DOI: 10.1056/NEJMoa0912614. PMID: 20525993.
Article5. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. 2013; Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 27:107–12. DOI: 10.1038/leu.2012.181. PMID: 22763385.
Article6. Novartis. 2010. Highlights of prescribing information: Tasigna (nilotinib) [package insert]. Novartis Pharma Stein;Stein, Switzerland: DOI: 10.1038/leu.2012.181.7. le Coutre PD, Giles FJ, Hochhaus A, et al. 2012; Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 26:1189–94. DOI: 10.1038/leu.2011.323. PMID: 22076466.
Article8. Baccarani M, Cortes J, Pane F, et al. 2009; Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 27:6041–51. DOI: 10.1200/JCO.2009.25.0779. PMID: 19884523. PMCID: PMC4979100.
Article9. Rosti G, Palandri F, Castagnetti F, et al. 2009; Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 114:4933–8. DOI: 10.1182/blood-2009-07-232595. PMID: 19822896.
Article10. Shin J, Koh Y, Yoon SH, et al. 2018; A phase 4 study of nilotinib in Korean patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTKorea. Cancer Med. 7:1814–23. DOI: 10.1002/cam4.1450. PMID: 29577674. PMCID: PMC5943463.
Article11. Hochhaus A, Saglio G, Hughes TP, et al. 2016; Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 30:1044–54. DOI: 10.1038/leu.2016.5. PMID: 26837842. PMCID: PMC4858585.
Article12. Baccarani M, Deininger MW, Rosti G, et al. 2013; European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 122:872–84. DOI: 10.1182/blood-2013-05-501569. PMID: 23803709. PMCID: PMC4915804.13. Kim DY, Lee JO, Kim KH, et al. 2015; Korean guidelines for treating chronic myelogenous leukemia - The Korean Society of Hematology Chronic Myelogenous Leukemia Working Party. Korean J Med. 88:406–19. DOI: 10.3904/kjm.2015.88.4.406.
Article14. Hochhaus A, Baccarani M, Silver RT, et al. 2020; European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 34:966–84. DOI: 10.1038/s41375-020-0776-2. PMID: 32127639. PMCID: PMC7214240.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nilotinib-Induced Acute Interstitial Nephritis during the Treatment of Chronic Myeloid Leukemia
- Metformin enhances the cytotoxic effect of nilotinib and overcomes nilotinib resistance in chronic myeloid leukemia cells
- Treatment-free remission after discontinuation of imatinib, dasatinib, and nilotinib in patients with chronic myeloid leukemia
- Problems within the post-marketing surveillance system in Korea: Time for a change
- Recent Advances of Management for Chronic Myeloid Leukemia