Kosin Med J.
2021 Dec;36(2):153-160.. 10.7180/kmj.2021.36.2.153.
Nilotinib-Induced Acute Interstitial Nephritis during the Treatment of Chronic Myeloid Leukemia
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea
- KMID: 2524659
- DOI: http://doi.org/10.7180/kmj.2021.36.2.153
Abstract
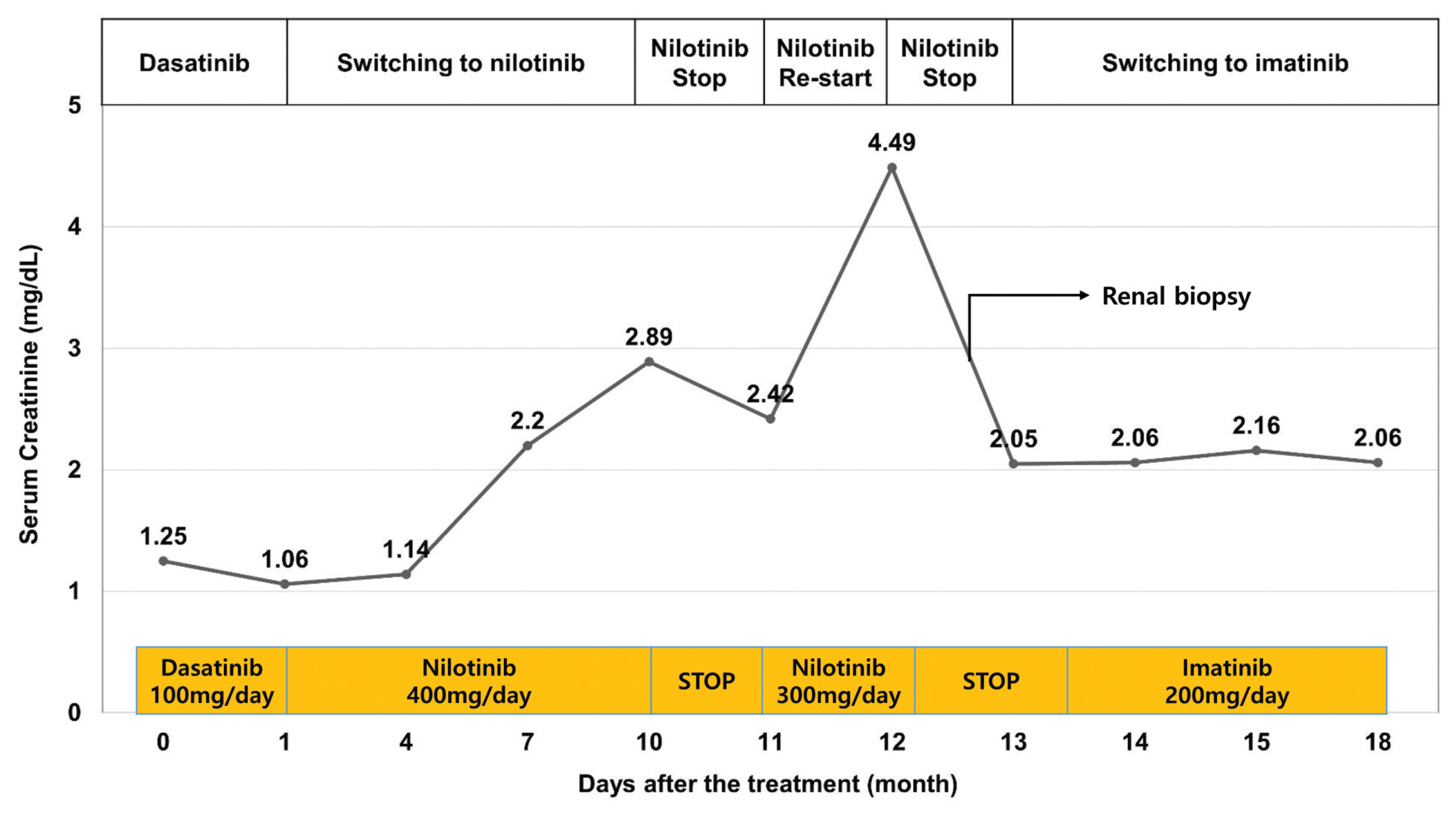
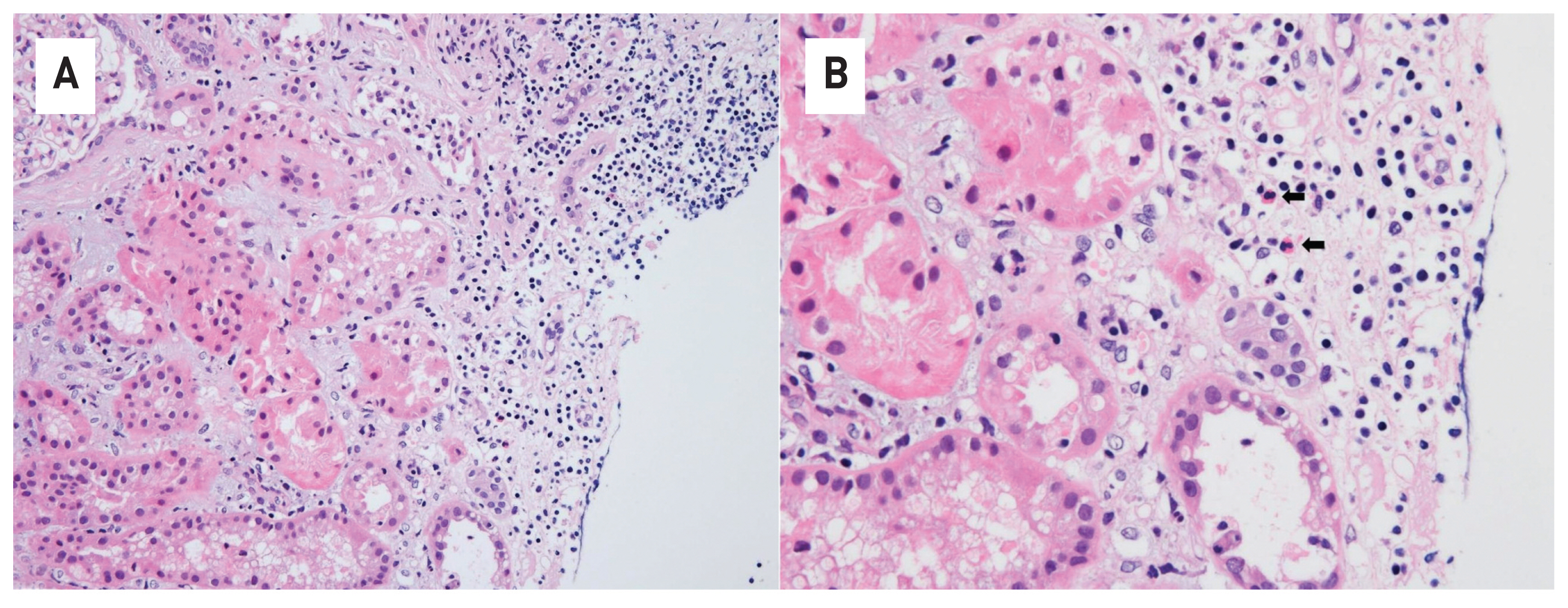
- Tyrosine kinase inhibitors (TKIs) are targeted therapy drugs that selectively inhibit protein kinases. Nephrotoxicity associated with TKIs is uncommon. We report a case of a 39-year-old man with acute kidney injury that developed after nilotinib treatment for chronic myeloid leukemia (CML). The renal function of the patient decreased during treatment with nilotinib but improved when treatment was discontinued due to neutropenia. However, the renal function of the patient deteriorated again with the reintroduction of nilotinib for treatment. A renal biopsy revealed acute interstitial nephritis (AIN). The patient had no history of comorbidities and medication causing renal injury. Finally, we diagnosed the patient with nilotinib-induced AIN. After switching to imatinib mesylate, the renal function of the patient stabilized without further deterioration. Our case indicates that nilotinib can be a potential cause of renal dysfunction by inducing AIN when renal function deteriorates in patients treated with nilotinib.
Figure
Reference
-
1. Jabbour E, Kantarjian H, Cortes J. Use of Second- and Third-Generation Tyrosine Kinase Inhibitors in the Treatment of Chronic Myeloid Leukemia: An Evolving Treatment Paradigm. Clinical Lymphoma Myeloma and Leukemia. 2015; 15:323–4.
Article2. Blay JY, von Mehren M. Nilotinib: a novel, selective tyrosine kinase inhibitor. Semin Oncol. 2011; 38:Suppl 1. S3–9.
Article3. Jhaveri KD, Wanchoo R, Sakhiya V, Ross DW, Fishbane S. Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review. Kidney Int Rep. 2016; 2:108–23.
Article4. Yilmaz M, Lahoti A, O’Brien S, Nogueras-Gonzáez GM, Burger J, Ferrajoli A, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer. 2015; 121:3894–904.
Article5. Ren X, Qin Y, Huang X, Zuo L, Jiang Q. Assessment of chronic renal injury in patients with chronic myeloid leukemia in the chronic phase receiving tyrosine kinase inhibitors. Ann Hematol. 2019; 98:1627–40.
Article6. Marcolino MS, Boersma E, Clementino NCD, Macedo AV, Marx-Neto AD, Silva M, et al. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol. 2011; 22:2073–9.
Article7. Jabbour E, Lipton JH. A critical review of trials of first-line BCR-ABL inhibitor treatment in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Clin Lymphoma Myeloma Leuk. 2013; 13:646–56.8. Ozkurt S, Temiz G, Acikalin MF, Soydan M. Acute renal failure under dasatinib therapy. Ren Fail. 2010; 32:147–9.
Article9. Hua J, Iwaki Y, Inoue M, Hagihara M. Tumor lysis syndrome soon after treatment with hydroxyurea followed by nilotinib in two patients with chronic-phase chronic myelogenous leukemia. Int J Hematol. 2013; 98:243–6.
Article10. Praga M, Sevillano A, Auńón P, Gonzáez E. Changes in the aetiology, clinical presentation and management of AIN, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant. 2015; 30:1472–9.11. Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009; 122:322–8.
Article12. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016; 90:638–47.
Article13. Shirali AC, Perazella MA, Gettinger S. Association of AIN with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016; 68:287–91.14. Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond interstitial nephritis: singlecenter experience. J Immunother Cancer. 2019; 7:2–14.
Article15. Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2018; 12:81–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin enhances the cytotoxic effect of nilotinib and overcomes nilotinib resistance in chronic myeloid leukemia cells
- A Case of Carbamazepine-Induced Acute Interstitial Nephritis
- Treatment-free remission after discontinuation of imatinib, dasatinib, and nilotinib in patients with chronic myeloid leukemia
- A Case of Acetaminophen-induced Acute Interstitial Nephritis Presenting with Acute Renal Failure
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy