J Korean Acad Oral Health.
2022 Jun;46(2):70-77. 10.11149/jkaoh.2022.46.2.70.
Lysozyme hydrochloride 0.01%, sodium fluoride 0.02%, cetylpyridinium chloride 0.05% antibacterial and sterilizing effect of mouth freshener
- Affiliations
-
- 1Department of Preventive Dentistry, College of Dentistry, Dankook University, Cheonan, Korea
- 2Aekyung Industrial Co., Ltd. R&D Division Dental Care Team, Daejeon, Korea
- KMID: 2530815
- DOI: http://doi.org/10.11149/jkaoh.2022.46.2.70
Abstract
Objectives
This study aimed to assess the antibacterial, bactericidal, and mouth freshener effects of lysozyme hydrochloride 0.01%, sodium fluoride 0.02%, and cetylpyridinium chloride 0.05%.
Methods
Eight oral disease-related bacteria were cultivated anaerobically. Four samples were prepared with or without 0.5% cetylpyridinium chloride, 0.2% sodium fluoride, and 0.1% lysozyme hydrochloride. Antimicrobial activity was tested in 96-well microplates. After assessing the bacterial count, the bacterial suspension was mixed with samples and spread on agar. The bactericidal rate was calculated by counting and comparing treated and untreated colonies.
Results
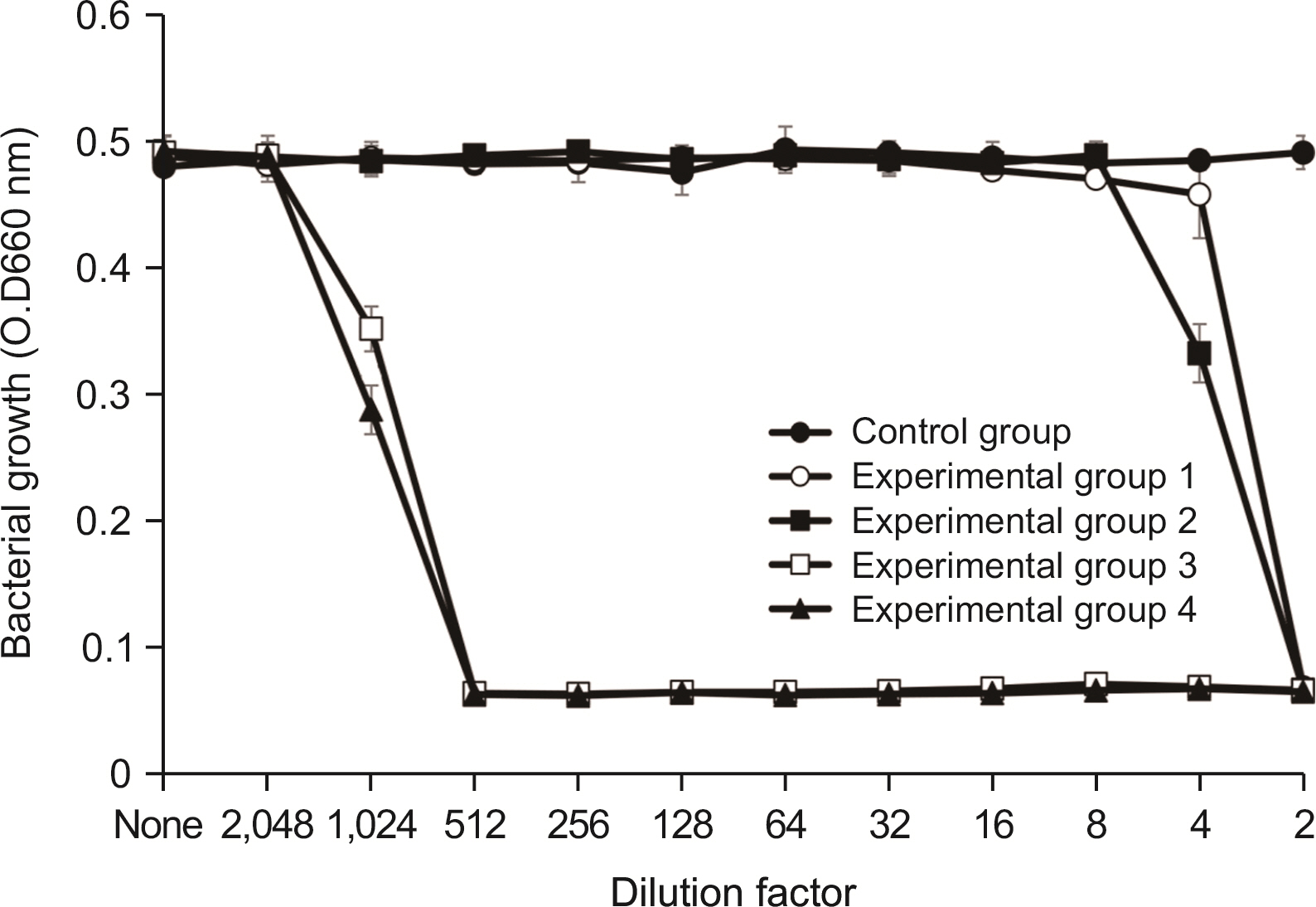
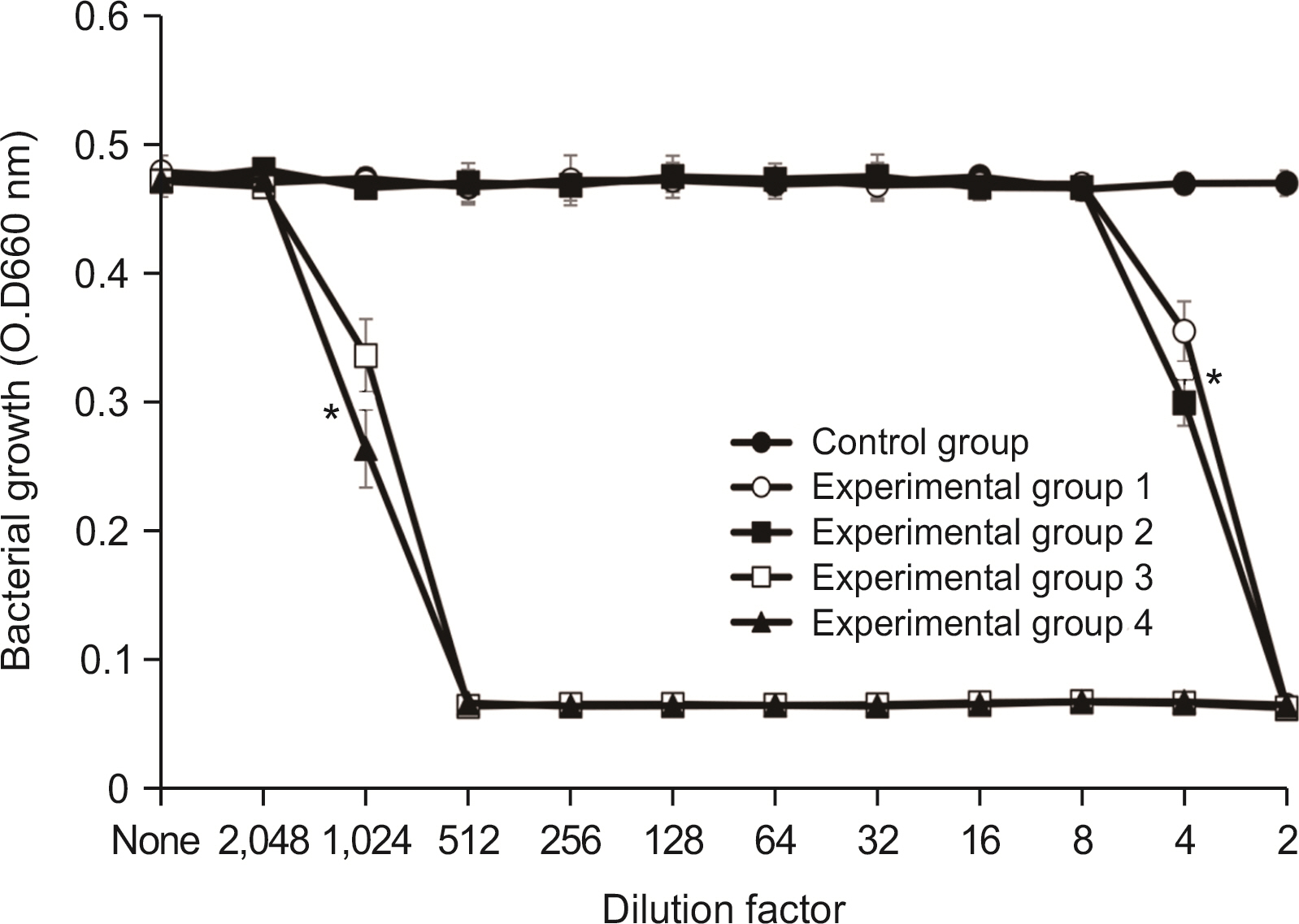
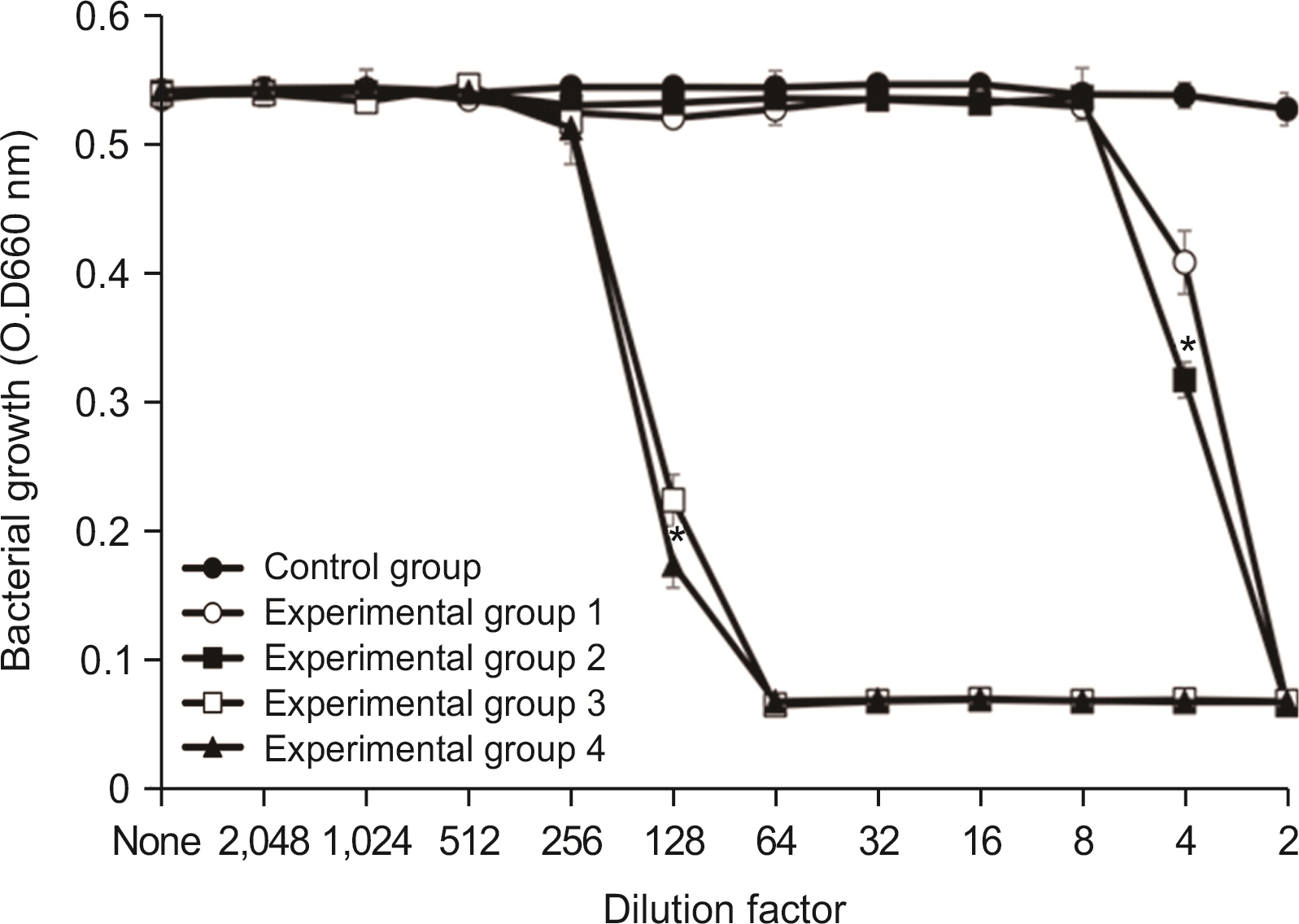
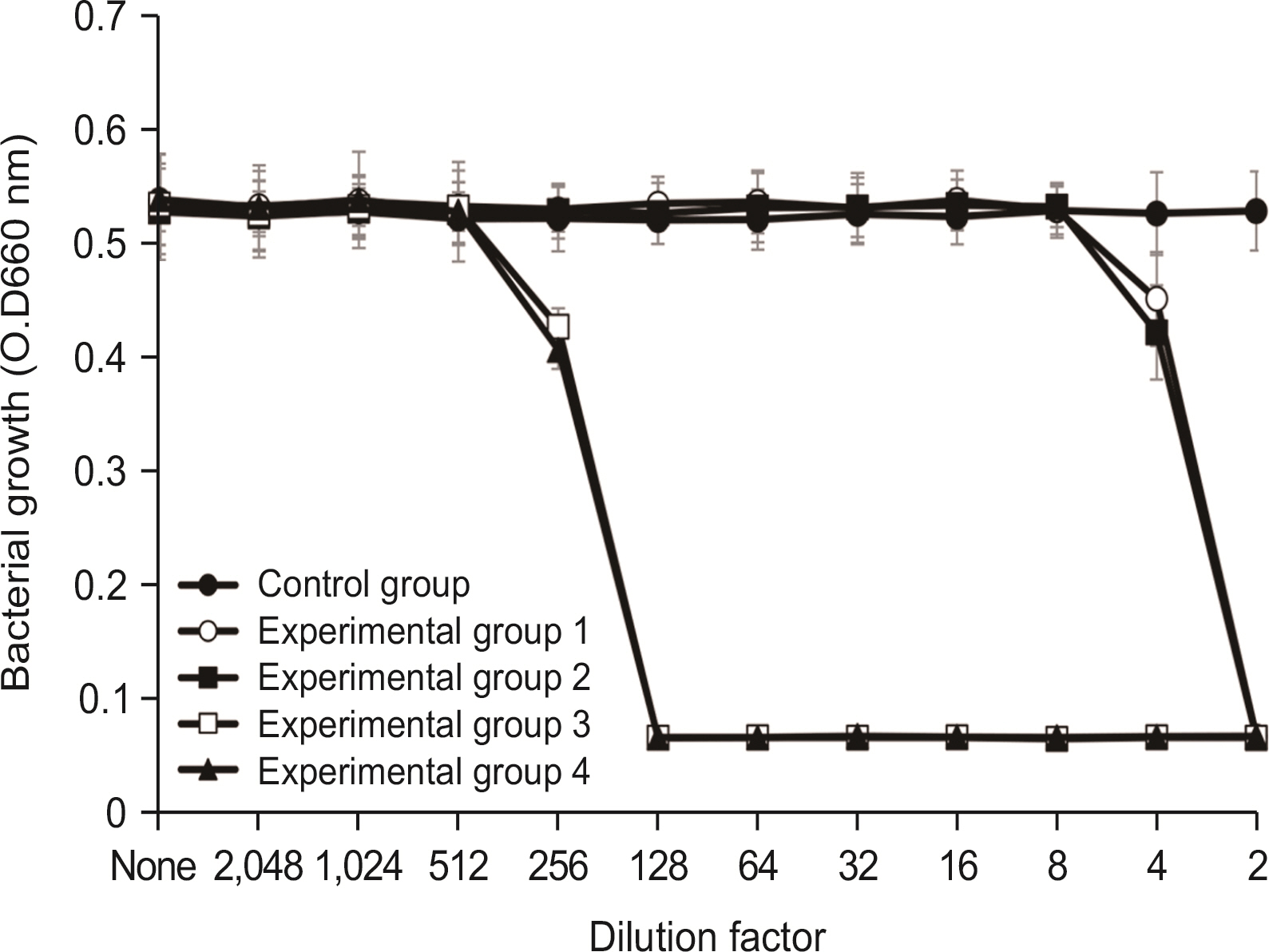
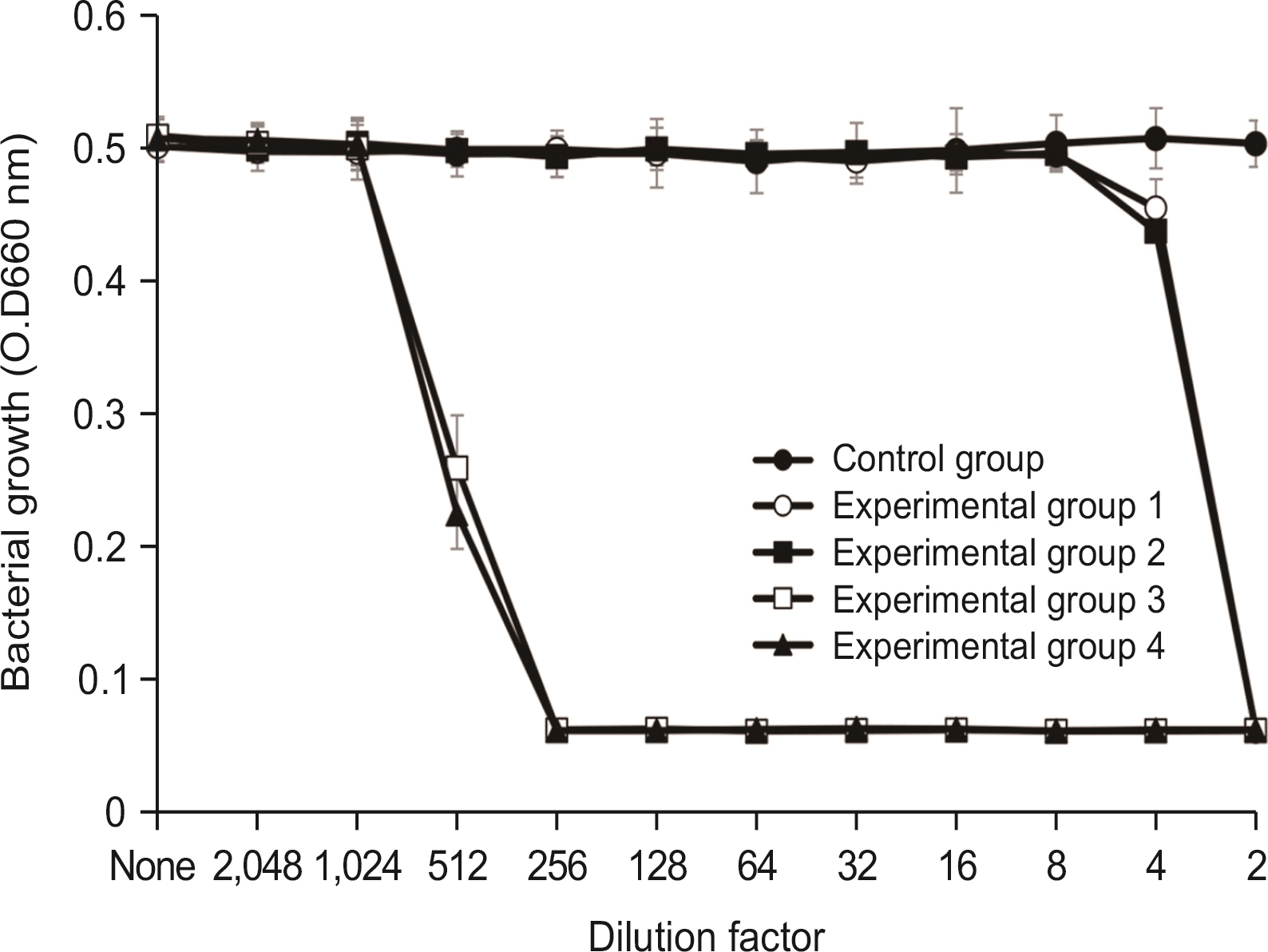
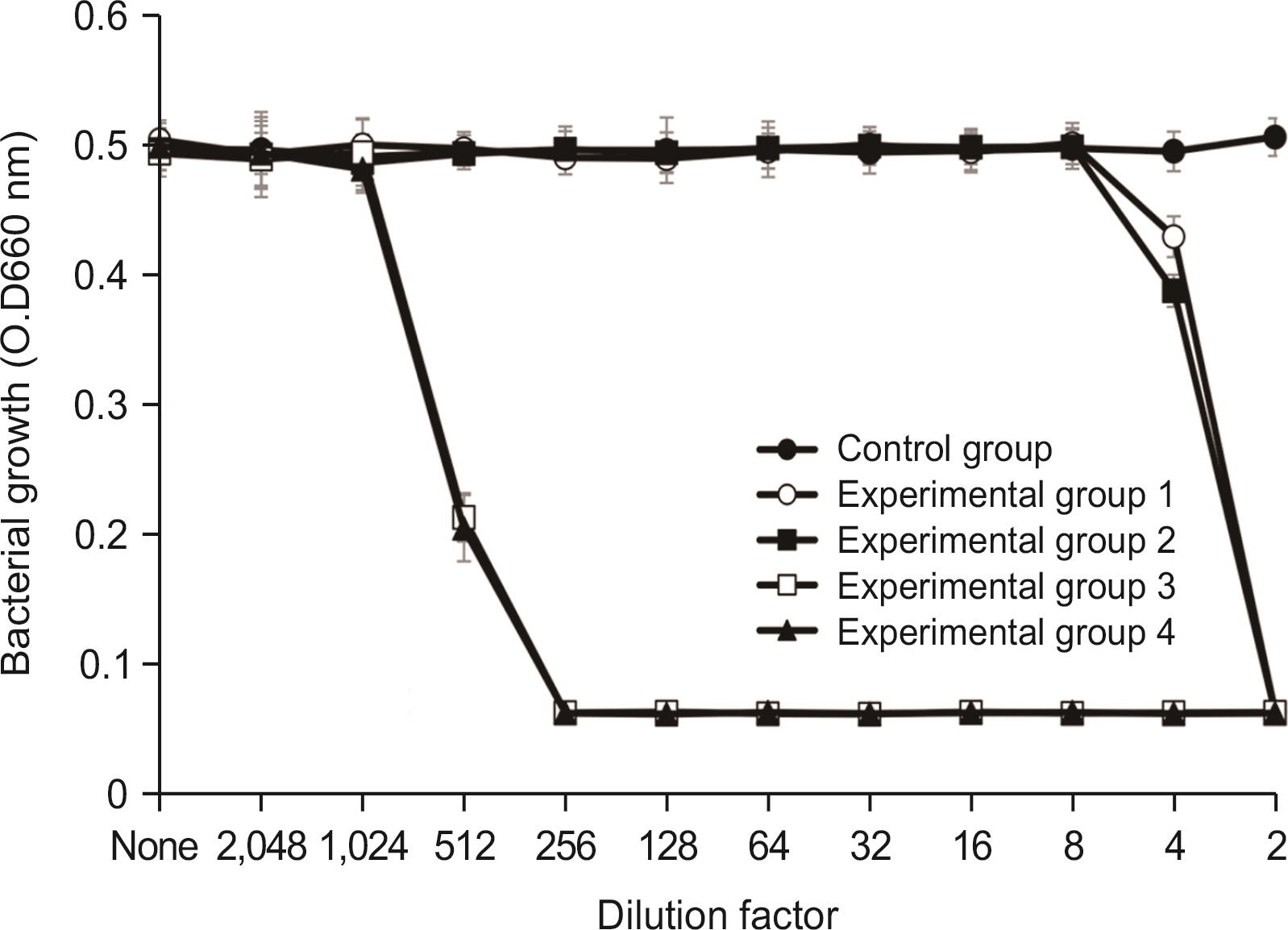
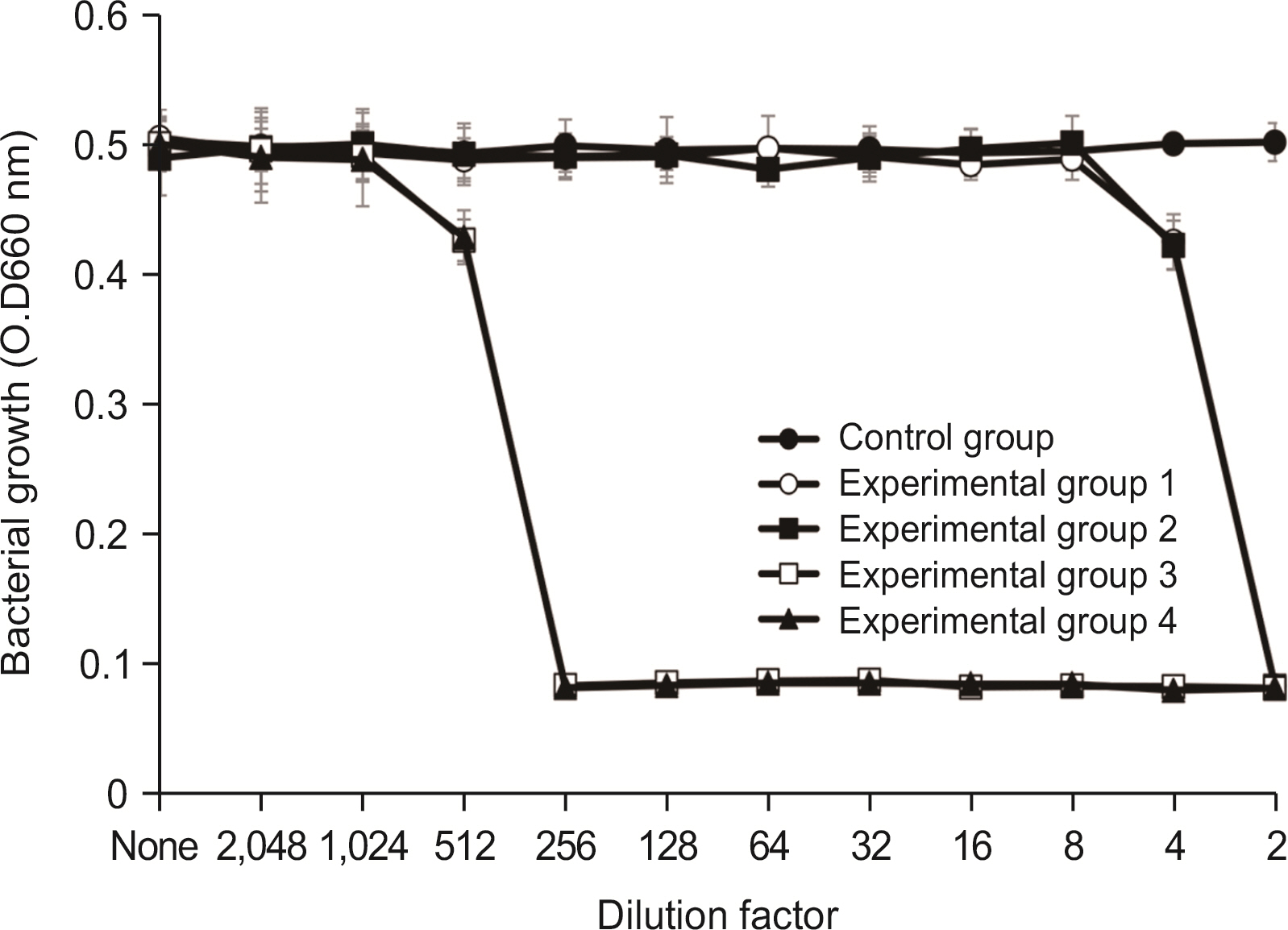
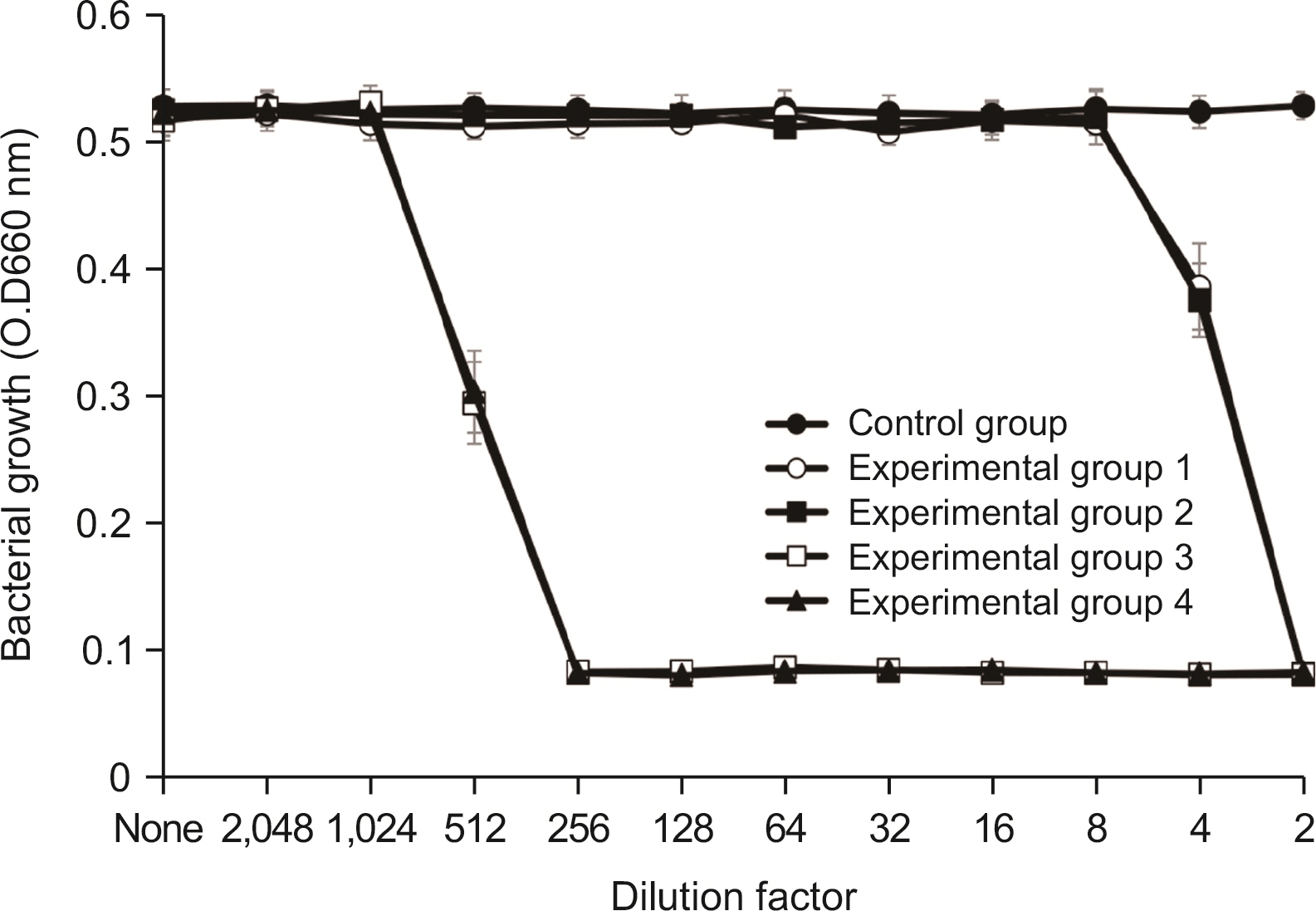
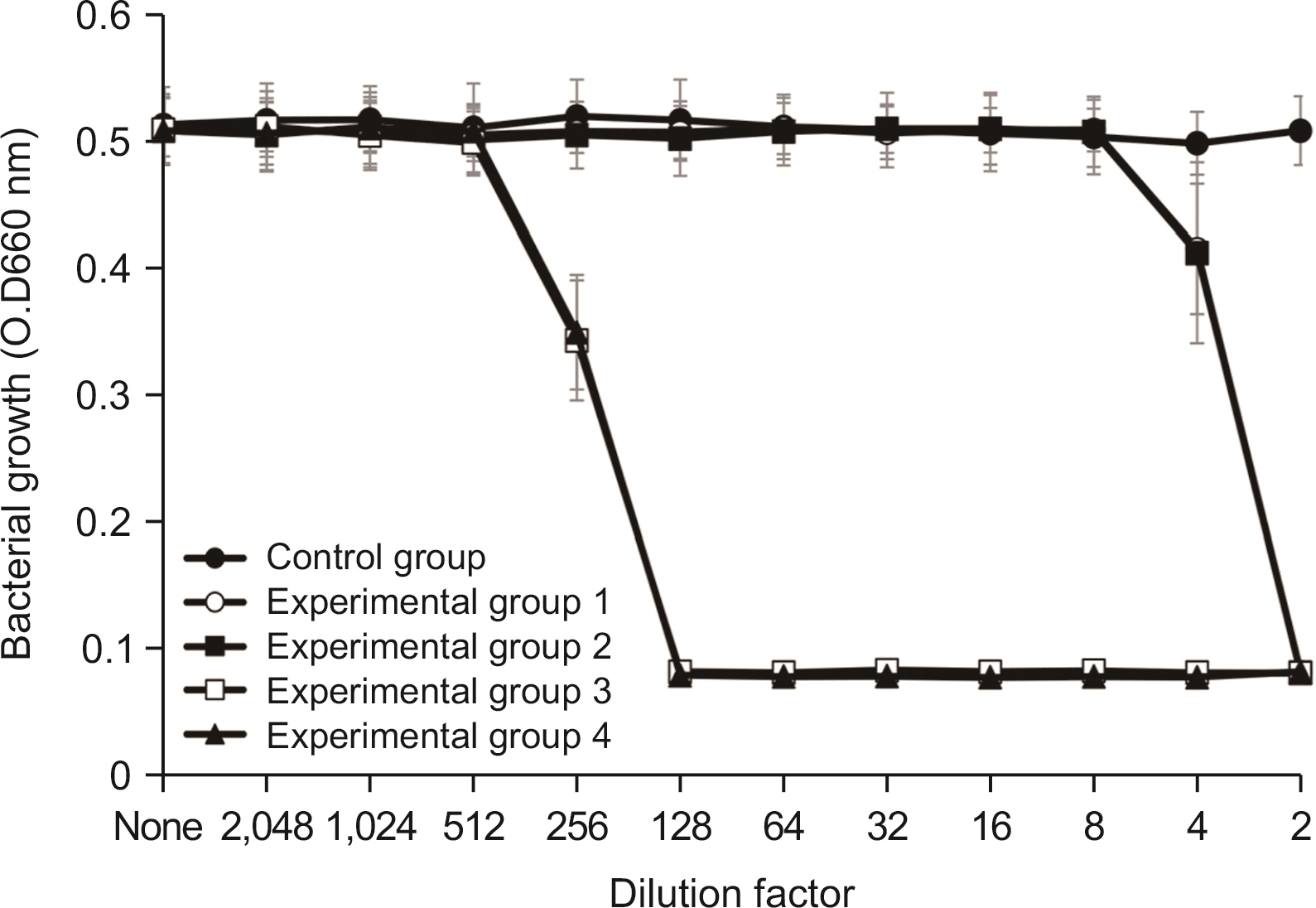
Lysozyme hydrochloride 0.01%, sodium fluoride 0.02%, and cetylpyridinium chloride 0.05% mouth fresheners sterilized 99.99% of 8 oral bacteria, including Streprococcus mutans. Lysozyme hydrochloride 0.01%, sodium fluoride 0.02%, and cetylpyridinium chloride 0.05% mouth fresheners showed 99.97% bactericidal activity against Lactobacillus acidophilus.
Conclusions
Lysozyme hydrochloride 0.01%, sodium fluoride 0.02%, and cetylpyridinium chloride 0.05% mouth fresheners confirmed the sterilization and antibacterial effects on oral disease-causing bacteria.
Figure
Reference
-
References
1. Marsh PD. 1994; Microbial ecology of dental plaque and its significance in health and disease. Advances in dental research. 8(2):263–71. DOI: 10.1177/08959374940080022001. PMID: 7865085. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028475512&origin=inward.
Article2. Kim TI, Choi EJ, Han SB. 2002; Antimicorbial effect of Zea Mays L. and Magnoliae cortex extract mixtures on periodontal pathogen and effect on human gingival fibroblast cellular activity. J of Periodontal &. Implant Sci. 32(1):249–55. DOI: 10.5051/jkape.2002.32.1.249.
Article3. Maeda H, Hirai K, Mineshiba J, Yamamoto T, Kokeguchi S, Takashiba S. 2013; Medical microbiological approach to Archaea in oral infectious diseases. Jpn Dent Sci Rev. 49(2):72–8. DOI: 10.1016/j.jdsr.2013.01.002. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876849866&origin=inward.
Article4. Robert P Allaker, Ian Douglas CW. 2009; Novel anti-microbial therapies for dental plaque related diseases. Int J Antimicrob Agent. 33(1):8–13. DOI: 10.1016/j.ijantimicag.2008.07.014. PMID: 18804350. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=59349085510&origin=inward.5. Park OJ, Kwon YK, Yun CH, Han SH. 2016; Augmented Osteoclastogenesis from Committed Osteoclast Precursors by Periodontopathic Bacteria Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis, Microbiol. Biotechnol. Lett. 44(4):557–62. DOI: 10.4014/mbl.1608.08010. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85012937613&origin=inward.6. Alireza RGA, Afsaneh R, Hosein MSS, Siamak Y, Afshin K, Zeinab K, Reza RA. 2014; Inhibitory activity of Salvadora persica extracts against oral bacterial strains associated with periodontitis: an in-vitro study. J Oral Biol Craniofac Res. 4(1):19–23. DOI: 10.1016/j.jobcr.2014.01.001. PMID: 25737914. PMCID: PMC4252377. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84928809205&origin=inward.
Article7. Shin SC, Lee H. 1998; Clinical study of mouth rinse containing sodium fluoride, cetylpyridinium chloride and urusodesoxycholinic acid on dental plaque and gingivitis. J Korean Acad Dent Health. 22:121–34.8. Gunsolley , John C. 2010; Clinical efficacy of antimicrobial mouthrinses. J of dent. 38(1):6–10. DOI: 10.1016/S0300-5712(10)70004-X. PMID: 20621242. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77955407233&origin=inward.
Article9. Fardal O, Tumbull RS. 1986; A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 112:863–9. DOI: 10.14219/jada.archive.1986.0118. PMID: 2940282. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0022735287&origin=inward.
Article10. Garcia-Godoy Franklin, Klukowska Malgorzata A, Zhang Yanhui H, Anastasia Kay, Cheng Richard, Gabbard Marsha, Coggan John, White Donald J. 2014; Comparative bioavailability and antimicrobial activity of cetylpyridinium chloride mouthrinses in vitro and in vivo. Am J Dent. 27(4):185–90. PMID: 25831600. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84908210027&origin=inward.11. Linka WA, Golenia E, Zgoda MM, Kołodziejczyk MK. 2014; The use of semi-synthetic polymers in the formulation of sucking and chewable tablets containing sage extract and zinc gluconate. Polim Med. 44(4):237–45. PMID: 25932905. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84930787307&origin=inward.12. Lim K, Mustapha A. 2007; Inhibition of Escherichia coli O157:H7, Listeria monocytogenes and Staphylococcus aureus on sliced roast beef by cetylpyridinium chloride and acidified sodium chlorite. Food Microbiol. 24:89–94. DOI: 10.1016/j.fm.2006.04.005. PMID: 16943099. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746728811&origin=inward.
Article13. Sakata M, Hiraiwa H, Morita M, Tsurumi M, Okazaki M, Masamura M, Koizumi K, Kishimoto E, Watanabe T. 1986; Effect of a dentifrice containing lysozyme chloride on periodontal disease. Nihon Shishubyo Gakkai Kaishi. 28(1):228–34. DOI: 10.2329/perio.28.228. PMID: 3459772. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0022680207&origin=inward.14. Ishikawa I, Okada H, Kamoi K, Miyashita H, Ueno K, Hara K, Hasegawa A, Yamada S, Murai S, Ikeda K, Nakamura J, Hori T, Ota N, NoguchiI T, Iwayama Y, Yamaoka A, Murayama Y, Okamoto H, Kuriyama K. 1992; Clinical Evaluation of Lysozyme Chloride on Marginal Periodontitis Using Double-Blind Comparative Study. Nihon Shishubyo Gakkai Kaishi. 34(4):883–900. DOI: 10.2329/perio.34.4_883.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical evaluation of cetylpyridinium chloride-containing mouthwash in halitosis
- Effects of a mouthwash containing potassium nitrate, sodium fluoride, and cetylpyridinium chloride on dentin hypersensitivity: a randomized, double-blind, placebo-controlled study
- The Short Time Antibacterial Effect of Tetracaine Hydrochloride(Pontocaine(R)): in vitro study
- Antibacterial effect of mouthwash containing CPC against dental caries caused bacteria
- Effect of Sodium Chloride on Biology of Catenaria anguillulae