J Stroke.
2022 May;24(2):236-244. 10.5853/jos.2021.01340.
Causal Relations between Exposome and Stroke: A Mendelian Randomization Study
- Affiliations
-
- 1Department of Neurology and Institute of Neurology, Huashan Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Shanghai Medical College, Fudan University, Shanghai, China
- 2Department of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
- 3Genetics and Aging Research Unit, McCance Center for Brain Health, Mass General Institute for Neurodegenerative Diseases (MIND), Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA
- 4Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China
- KMID: 2530526
- DOI: http://doi.org/10.5853/jos.2021.01340
Abstract
- Background and Purpose
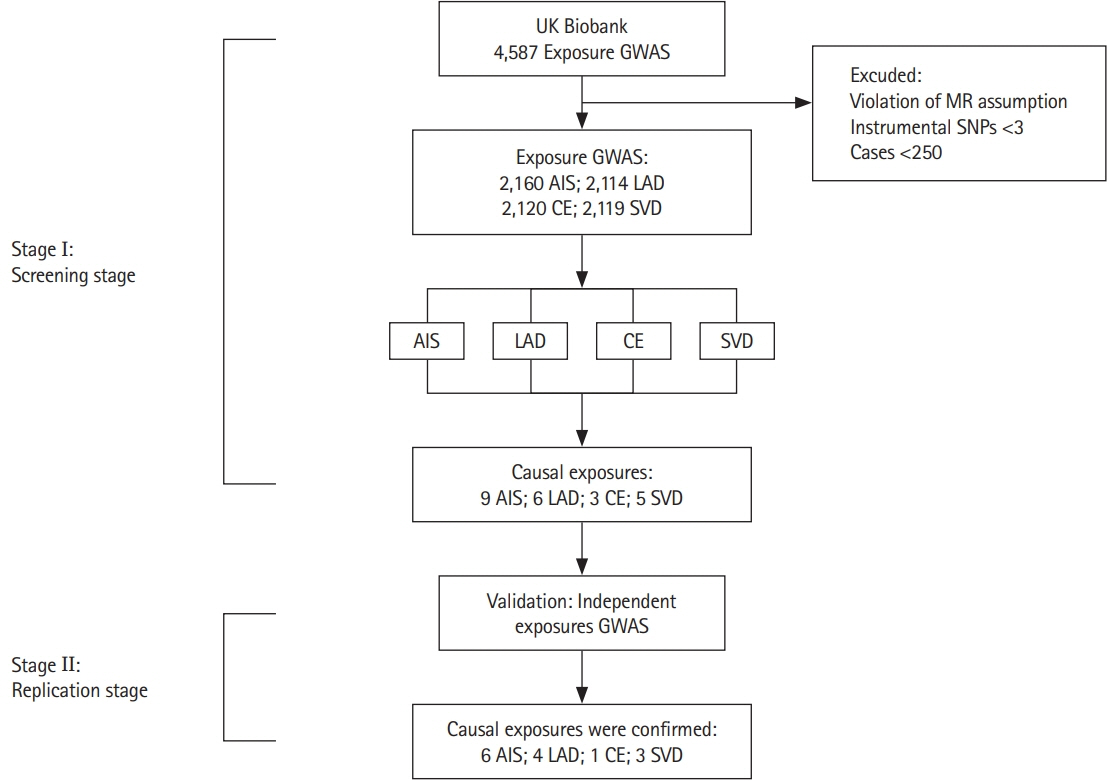
To explore the causal relationships of elements of the exposome with ischemic stroke and its subtypes at the omics level and to provide evidence for stroke prevention. Methods We conducted a Mendelian randomization study between exposure and any ischemic stroke (AIS) and its subtypes (large-artery atherosclerotic disease [LAD], cardioembolic stroke [CE], and small vessel disease [SVD]). The exposure dataset was the UK Biobank involving 361,194 subjects, and the outcome dataset was the MEGASTROKE consortium including 52,000 participants.
Results
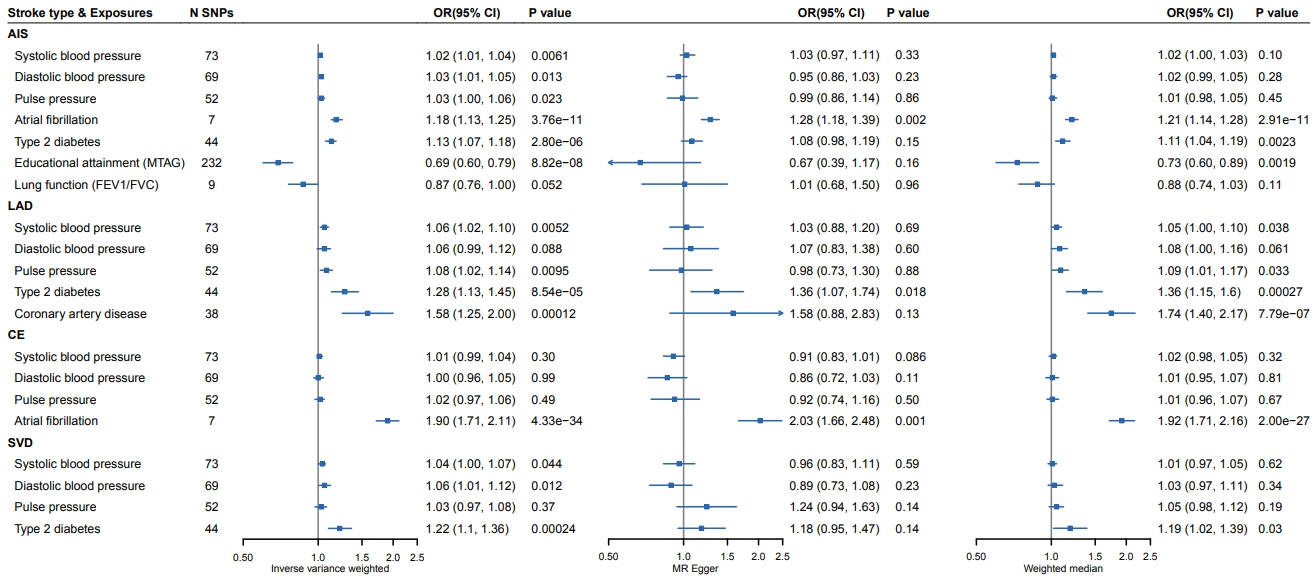
We found that higher blood pressure (BP) (systolic BP: odds ratio [OR], 1.02; 95% confidence interval [CI], 1.01 to 1.04; diastolic BP: OR, 1.03; 95% CI, 1.01 to 1.05; pulse pressure: OR, 1.03; 95% CI, 1.00 to 1.06), atrial fibrillation (OR, 1.18; 95% CI, 1.13 to 1.25), and diabetes (OR, 1.13; 95% CI, 1.07 to 1.18) were significantly associated with ischemic stroke. Importantly, higher education (OR, 0.69; 95% CI, 0.60 to 0.79) decreased the risk of ischemic stroke. Higher systolic BP (OR, 1.06; 95% CI, 1.02 to 1.10), pulse pressure (OR, 1.08; 95% CI, 1.02 to 1.14), diabetes (OR, 1.28; 95% CI, 1.13 to 1.45), and coronary artery disease (OR, 1.58; 95% CI, 1.25 to 2.00) could cause LAD. Atrial fibrillation could cause CE (OR, 1.90; 95% CI, 1.71 to 2.11). For SVD, higher systolic BP (OR, 1.04; 95% CI, 1.00 to 1.07), diastolic BP (OR, 1.06; 95% CI, 1.01 to 1.12), and diabetes (OR, 1.22; 95% CI, 1.10 to 1.36) were causal factors.
Conclusions
The study revealed elements of the exposome causally linked to ischemic stroke and its subtypes, including conventional causal risk factors and novel protective factors such as higher education.
Keyword
Figure
Reference
-
References
1. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016; 388:761–775.
Article2. GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018; 379:2429–2437.
Article3. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016; 15:913–924.
Article4. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018; 362:k601.
Article5. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. 2017; 4:330–345.
Article6. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005; 14:1847–1850.
Article7. Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, Miller GW. The exposome: molecules to populations. Annu Rev Pharmacol Toxicol. 2019; 59:107–127.
Article8. Nieuwenhuijsen MJ, Agier L, Basagaña X, Urquiza J, Tamayo-Uria I, Giorgis-Allemand L, et al. Influence of the urban exposome on birth weight. Environ Health Perspect. 2019; 127:47007.
Article9. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018; 562:203–209.
Article10. Finch CE, Kulminski AM. The Alzheimer’s disease exposome. Alzheimers Dement. 2019; 15:1123–1132.
Article11. Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012; 41:24–32.
Article12. Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011; 21:5–9.
Article13. Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014; 69:876–878.
Article14. Doherty A, Smith-Byrne K, Ferreira T, Holmes MV, Holmes C, Pulit SL, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. 2018; 9:5257.
Article15. Noyce AJ, Bandres-Ciga S, Kim J, Heilbron K, Kia D, Hemani G, et al. The Parkinson’s disease Mendelian randomization research portal. Mov Disord. 2019; 34:1864–1872.
Article16. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018; 50:524–537.17. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018; 50:1412–1425.18. Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012; 44:670–675.19. Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018; 9:2941.
Article20. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018; 50:1112–1121.21. Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017; 49:416–425.22. Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015; 47:381–386.
Article23. Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet. 2017; 49:1385–1391.
Article24. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40:304–314.
Article25. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015; 44:512–525.
Article26. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011; 40:740–752.
Article27. Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014; 43:922–929.
Article28. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018; 7:e34408.
Article29. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016; 45:1717–1726.
Article30. Jackson CA, Sudlow CL, Mishra GD. Education, sex and risk of stroke: a prospective cohort study in New South Wales, Australia. BMJ Open. 2018; 8:e024070.
Article31. Gillum RF, Mussolino ME. Education, poverty, and stroke incidence in whites and blacks: the NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol. 2003; 56:188–195.
Article32. Hankey GJ. Stroke. Lancet. 2017; 389:641–654.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impaired pulmonary function mediates the impact of preterm birth on later-life stroke: a 2-step, multivariable Mendelian randomization study
- The mediating role of atrial fibrillation in causal associations between risk factors and stroke: a Mendelian randomization study
- Clinical and genetic relationships between the QTc interval and risk of a stroke among atrial fibrillation patients undergoing catheter ablation
- Exploration of errors in variance caused by using the first-order approximation in Mendelian randomization
- Basic Concepts of a Mendelian Randomization Approach