Int J Stem Cells.
2022 May;15(2):217-226. 10.15283/ijsc21136.
Effects and Mechanisms of Bone Marrow Mesenchymal Stem Cell Transplantation for Treatment of Ischemic Stroke in Hypertensive Rats
- Affiliations
-
- 1Department of Rehabilitation Medicine, Panyu Central Hospital, Guangzhou, China
- 2South China Normal University-Panyu Central Hospital Joint Laboratory of Translational Medical Research, Panyu Central Hospital, Guangzhou, China
- 3School of Life Sciences, South China Normal University, Guangzhou, China
- KMID: 2529836
- DOI: http://doi.org/10.15283/ijsc21136
Abstract
- Background and Objectives
Stroke is the most common cause of human death and functional disability, resulting in more than 5 million deaths worldwide each year. Bone marrow mesenchymal stem cells (BMSCs) are a kind of stem cell that are able to self-renew and differentiate into many types of tissues. Therefore, BMSCs have the potential to replace damaged neurons and promote the reconstruction of nerve conduction pathways and connective tissue. However, it remains unknown whether transplanted BMSCs promote angiogenesis or improve the tissue microenvironment directly or indirectly through paracrine interactions. This study aimed to determine the therapeutic effect of BMSCs on ischemic stroke with hypertension in a rodent model and to explore the possible mechanisms underlying any benefits.
Methods and Results
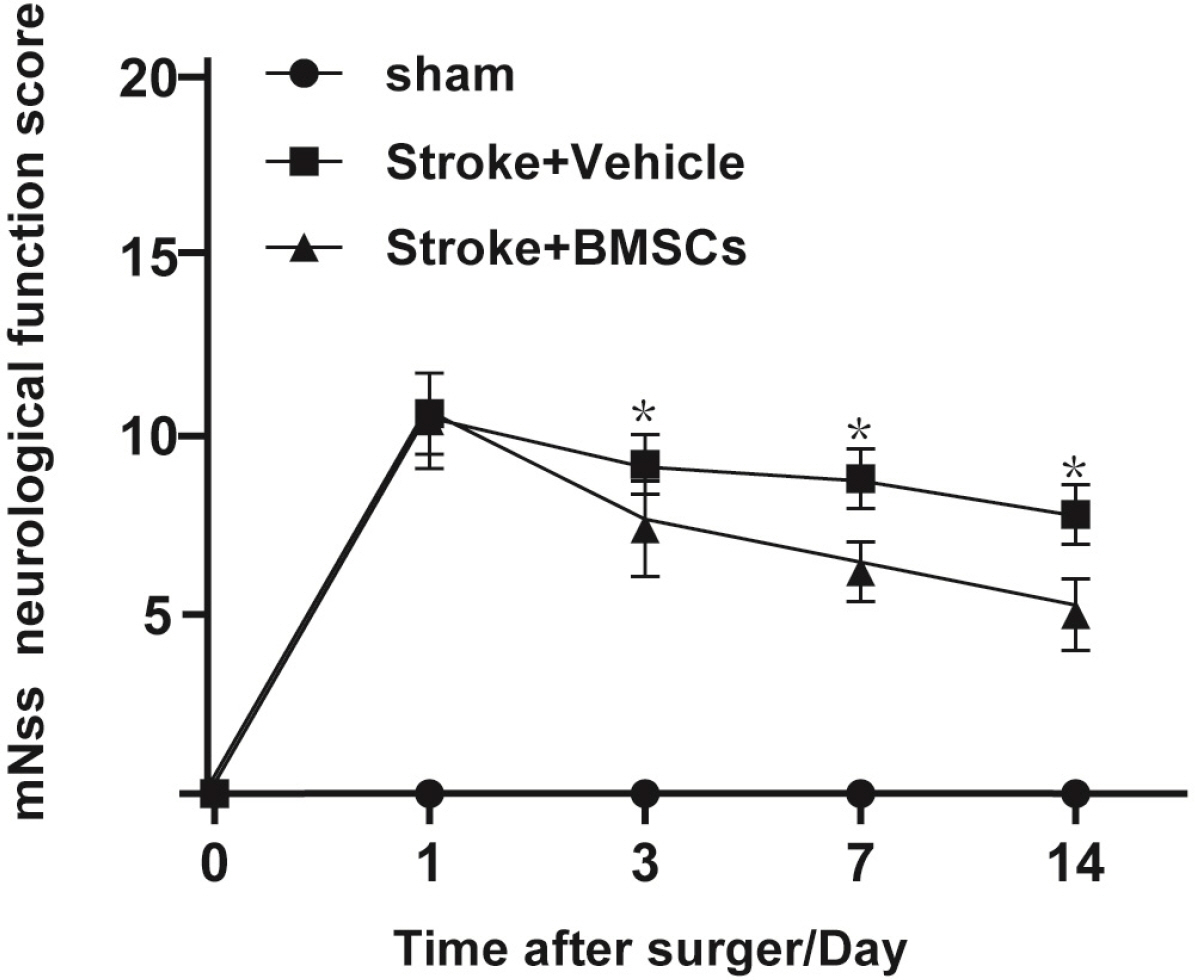
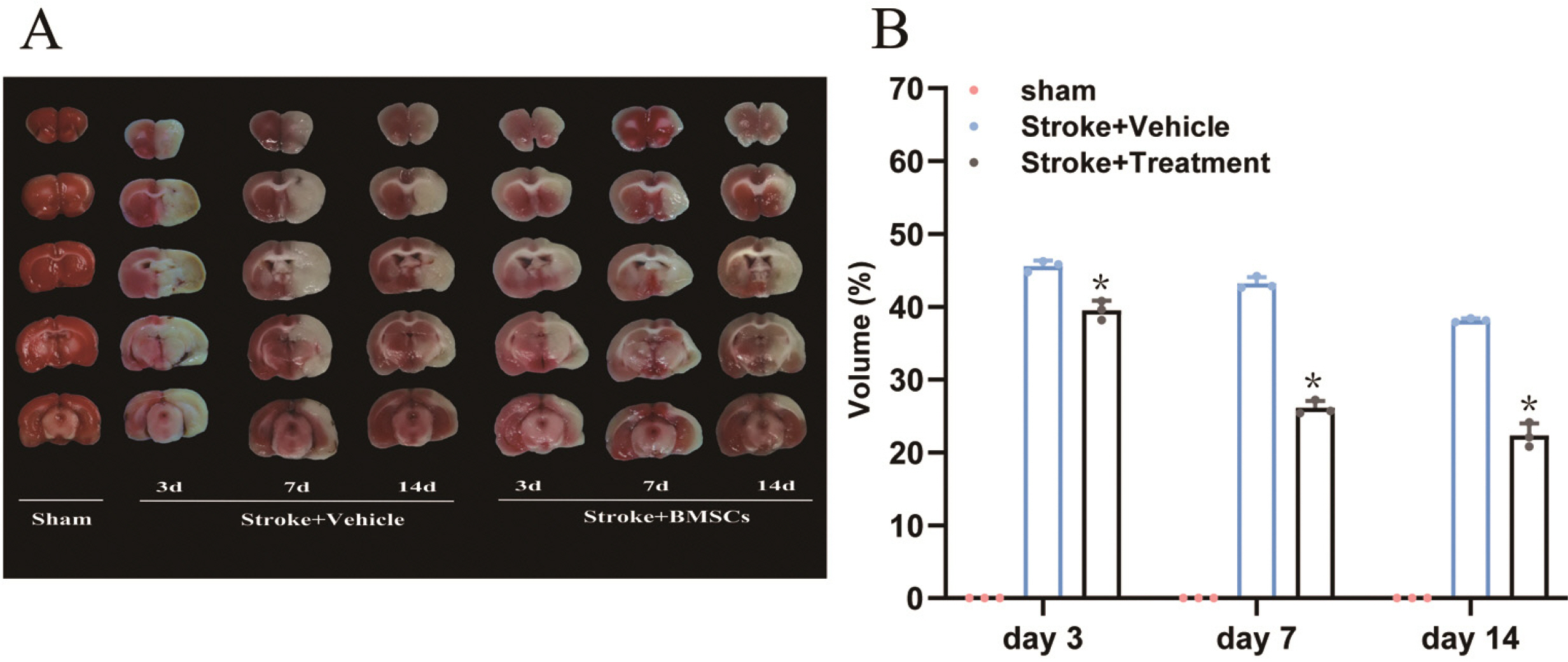
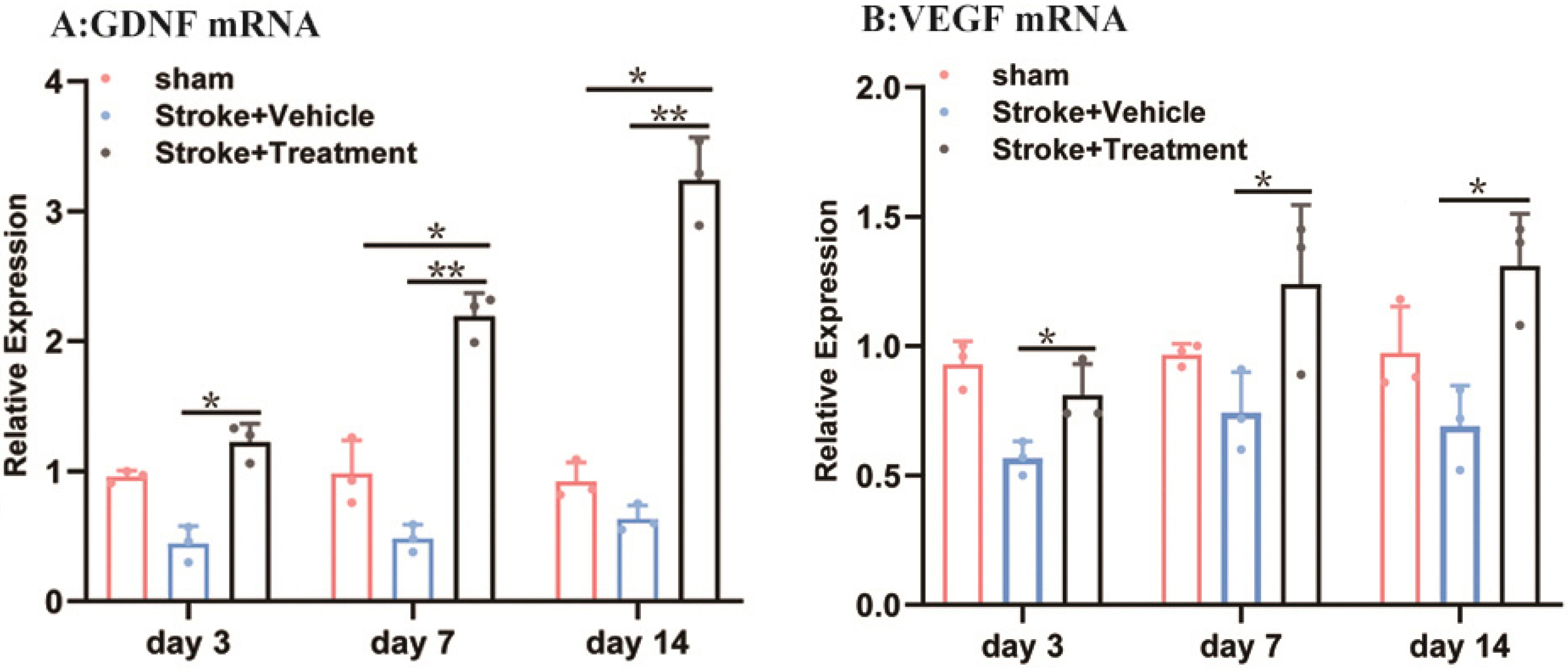
Middle cerebral artery occlusion was used to establish the experimental stroke model. The area of cerebral infarction, expression of vascular endothelial growth factor (VEGF) and glial cell line-derived neurotrophic factor (GDNF), and increment of astrocyte were measured by TTC staining, western blot, real-time quantitative polymerase chain reaction (RT-qPCR) and immunocytochemistry. The results showed a smaller area of cerebral infarction and improved neurological function scores in animals treated with BMSCs compared to controls. The results of RT-qPCR and western blot assays showed higher expression of VEGF and GDNF in BMSC-treated animals compared with controls. Our study also showed that one round of BMSCs transplantation significantly promoted the proliferation of subventricular zone and cortical cells, especially astrocytes, on the ischemic side following cerebral ischemia.
Conclusions
Above findings support that BMSCs have therapeutic effects for ischemic stroke complicated with hypertension, which may occur via up-regulated expression of VEGF and GDNF and reduction of neuronal apoptosis, thereby promoting the recovery of nerve function.
Figure
Reference
-
References
1. Camen S, Haeusler KG, Schnabel RB. 2020; Cardiac imaging after ischemic stroke or transient ischemic attack. Curr Neurol Neurosci Rep. 20:36. DOI: 10.1007/s11910-020-01053-3. PMID: 32607785. PMCID: PMC7326893.
Article2. Churilov L, Fridriksdottir A, Keshtkaran M, Mosley I, Flitman A, Dewey HM. 2013; Decision support in pre-hospital stroke care operations: a case of using simulation to improve eligibility of acute stroke patients for thrombolysis treatment. Comput Oper Res. 40:2208–2218. DOI: 10.1016/j.cor.2012.06.012.
Article3. Fisher M, Garcia JH. 1996; Evolving stroke and the ischemic penumbra. Neurology. 47:884–888. DOI: 10.1212/WNL.47.4.884. PMID: 8857713.
Article4. Xie W, Zhao ZH, Yang QM, Wei FH. 2015; The efficacy of the seamless transfer of care model to apply for the patients with cerebral apoplexy in China. Int J Nurs Sci. 2:52–57. DOI: 10.1016/j.ijnss.2015.01.012.
Article5. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. 2003; Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 73:778–786. DOI: 10.1002/jnr.10691. PMID: 12949903.
Article6. Chopp M, Li Y. 2002; Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1:92–100. DOI: 10.1016/S1474-4422(02)00040-6. PMID: 12849513.
Article7. De Keyser J. 2005; Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 58:653–654. author reply 654–655. DOI: 10.1002/ana.20612. PMID: 16178021.
Article8. Li M, Cui Z, Niu Y, Liu B, Fan W, Yu D, Deng J. 2010; Synaptogenesis in the developing mouse visual cortex. Brain Res Bull. 81:107–113. DOI: 10.1016/j.brainresbull.2009.08.028. PMID: 19751806.
Article9. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. 2002; Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 59:514–523. DOI: 10.1212/WNL.59.4.514. PMID: 12196642.
Article10. Cízková D, Rosocha J, Vanický I, Jergová S, Cízek M. 2006; Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol. 26:1167–1180. DOI: 10.1007/s10571-006-9093-1. PMID: 16897366.
Article11. Badr AE, Yin W, Mychaskiw G, Zhang JH. 2001; Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol. 280:R766–R770. DOI: 10.1152/ajpregu.2001.280.3.R766. PMID: 11171656.
Article12. Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. 2008; Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 17:1045–1059. DOI: 10.3727/096368908786991551. PMID: 19177841.
Article13. Ullrich E, Bosch J, Aigner M, Völkl S, Dudziak D, Spriewald B, Schuler G, Andreesen R, Mackensen A. 2010; Advances in cellular therapy: 5th International Symposium on the clinical use of cellular products, 19 and 20 March 2009, Nürnberg, Germany. Cancer Immunol Immunother. 59:1745–1756. DOI: 10.1007/s00262-009-0779-3. PMID: 19862524.
Article14. Baksh D, Song L, Tuan RS. 2004; Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 8:301–316. DOI: 10.1111/j.1582-4934.2004.tb00320.x. PMID: 15491506. PMCID: PMC6740223.
Article15. Jeong H, Yim HW, Cho YS, Kim YI, Jeong SN, Kim HB, Oh IH. 2014; Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 7:63–69. DOI: 10.15283/ijsc.2014.7.2.63. PMID: 25473443. PMCID: PMC4249905.
Article16. Yu H, Xu Z, Qu G, Wang H, Lin L, Li X, Xie X, Lei Y, He X, Chen Y, Li Y. 2021; Hypoxic preconditioning enhances the efficacy of mesenchymal stem cells-derived conditioned medium in switching microglia toward anti-inflammatory polarization in ischemia/reperfusion. Cell Mol Neurobiol. 41:505–524. DOI: 10.1007/s10571-020-00868-5. PMID: 32424775.
Article17. V Dzau M Mirotsou . Duke University. 2014. Jan. 29. Stem cell derived paracrine factor h1 for use in reducing cell death in cardiac tissue. EP2548569B1.18. Zhang LL, Zhang HT, Cai YQ, Han YJ, Yao F, Yuan ZH, Wu BY. 2016; Anti-inflammatory effect of mesenchymal stromal cell transplantation and quercetin treatment in a rat model of experimental cerebral ischemia. Cell Mol Neurobiol. 36:1023–1034. DOI: 10.1007/s10571-015-0291-6. PMID: 27008429.
Article19. Deng QJ, Xu XF, Ren J. 2021; Correction to: effects of SDF-1/CXCR4 on the repair of traumatic brain injury in rats by mediating bone marrow derived mesenchymal stem cells. Cell Mol Neurobiol. 41:617–618. DOI: 10.1007/s10571-020-00932-0. PMID: 32876898.
Article20. Bejan-Angoulvant T, Saadatian-Elahi M, Wright JM, Schron EB, Lindholm LH, Fagard R, Staessen JA, Gueyffier F. 2010; Treatment of hypertension in patients 80 years and older: the lower the better? A meta-analysis of randomized controlled trials. J Hypertens. 28:1366–1372. DOI: 10.1097/HJH.0b013e328339f9c5. PMID: 20574244.
Article21. Fang J, Tang XM, Wang S, Zheng B, Li ZX, Sun XH, Wu J, Fang J, Lu Q, Dong YR, Zhai Y, Zhang X, Pan H, Liu JR. 2015; Efficacy and safety of combined therapy of intravenous thrombolysis and endovascular thrombectomy in patients with acute ischemic stroke. Chin J Clin Neurosci. 23:481–487.22. Nejadnik H. 2013. Bone marrow derived mesenchymal stem cell (BM MSC) application in articular cartilage repair [PhD dissertation]. National University of Singapore;Singapore:23. Seo JH, Cho SR. 2012; Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med J. 53:1059–1067. DOI: 10.3349/ymj.2012.53.6.1059. PMID: 23074102. PMCID: PMC3481376.
Article24. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. 2002; Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 20:530–541. DOI: 10.1634/stemcells.20-6-530. PMID: 12456961.
Article25. Lei H, Lei L, Dong A, Hong T, Zhang Y, Zhou H, Song C, Mou C. 2017; Transplantation of bone marrow mesenchymal stem cells in the brain of rats with cerebral ischemia-reperfusion injury decreases expression of TNF-α and increases expression of TGFβ1. Chin J Histochem Cytochem. 26:327–335.26. Yang Y, Rosenberg GA. 2011; Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 42:3323–3328. DOI: 10.1161/STROKEAHA.110.608257. PMID: 21940972. PMCID: PMC3584169.
Article27. Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. 2014; Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 11:18. DOI: 10.1186/2045-8118-11-18. PMID: 25120903. PMCID: PMC4130123.
Article28. Wang C, Fei Y, Xu C, Zhao Y, Pan Y. 2015; Bone marrow mesenchymal stem cells ameliorate neurological deficits and blood-brain barrier dysfunction after intracerebral hemorrhage in spontaneously hypertensive rats. Int J Clin Exp Pathol. 8:4715–4724. PMID: 26191161. PMCID: PMC4503033.29. Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. 2011; Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 42:740–744. DOI: 10.1161/STROKEAHA.110.607226. PMID: 21307396. PMCID: PMC3060040.
Article30. Song M, Mohamad O, Gu X, Wei L, Yu SP. 2013; Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 22:2001–2015. DOI: 10.3727/096368912X657909. PMID: 23069268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- Stem Cells and Niemann Pick Disease
- Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model