Int J Stem Cells.
2022 May;15(2):183-194. 10.15283/ijsc21062.
Evaluation of the Potential Effects of Retinol and Alginate/Gelatin-Based Scaffolds on Differentiation Capacity of Mouse Mesenchymal Stem Cells (MSCs) into Retinal Cells
- Affiliations
-
- 1Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 2Department of Biology, Applied Biology Research Center, Mashhad Branch, Islamic Azad University, Mashhad, Iran
- 3Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran
- KMID: 2529790
- DOI: http://doi.org/10.15283/ijsc21062
Abstract
- Background and Objectives
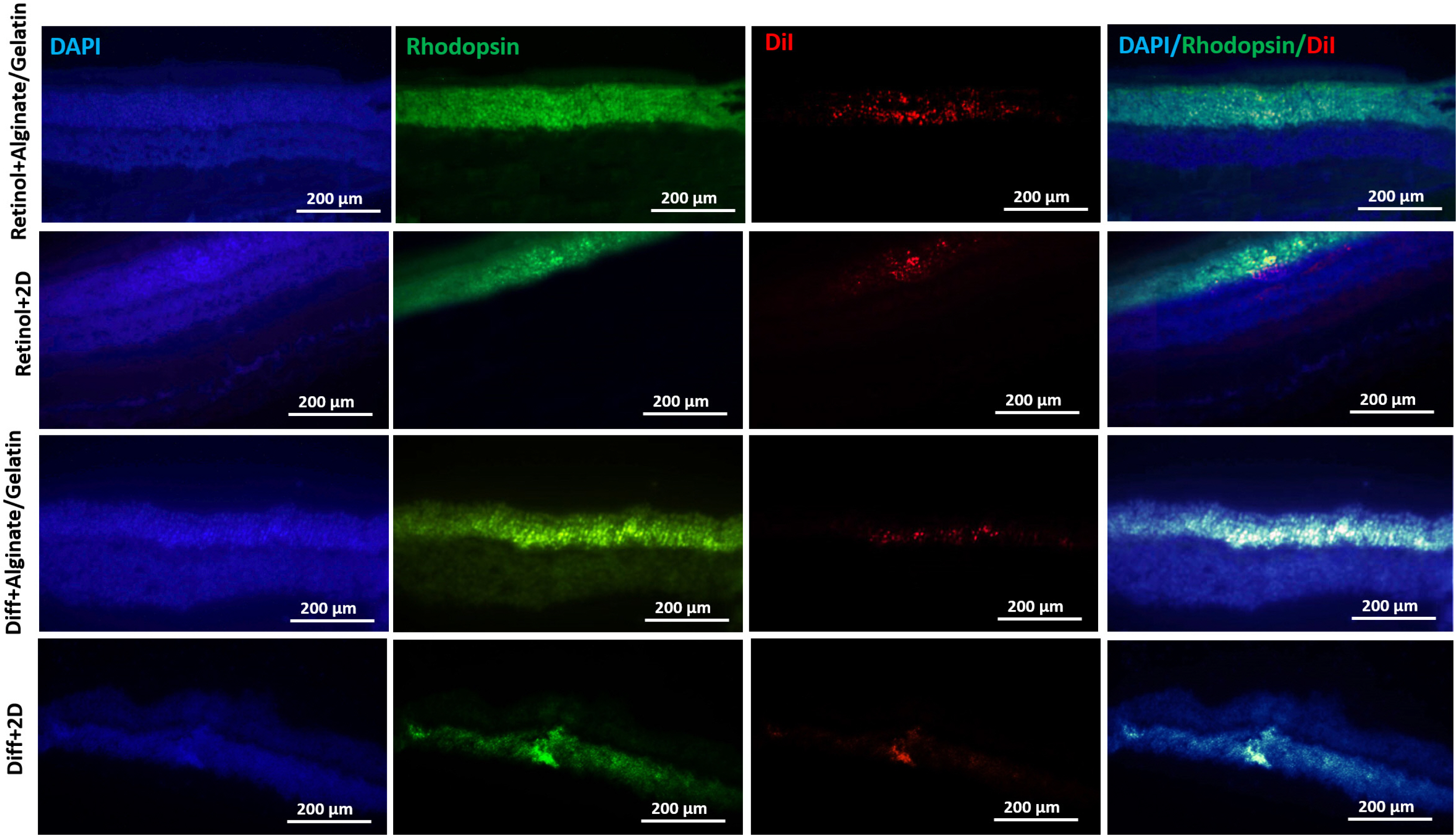
Retinal stem cells (RSCs) resided in ciliary epithelium have shown to possess a high ca-pacity to self-renew and differentiate into retinal cells. RSCs could be induced to differentiate when they are exposed to stimuli like natural compounds and suitable contexts such as biomaterials. The aim of this study was to examine the effects of Retinol and alginate/gelatin-based scaffolds on differentiation potential of mesenchymal stem cells (MSCs) originated from mouse ciliary epithelium.
Methods and Results
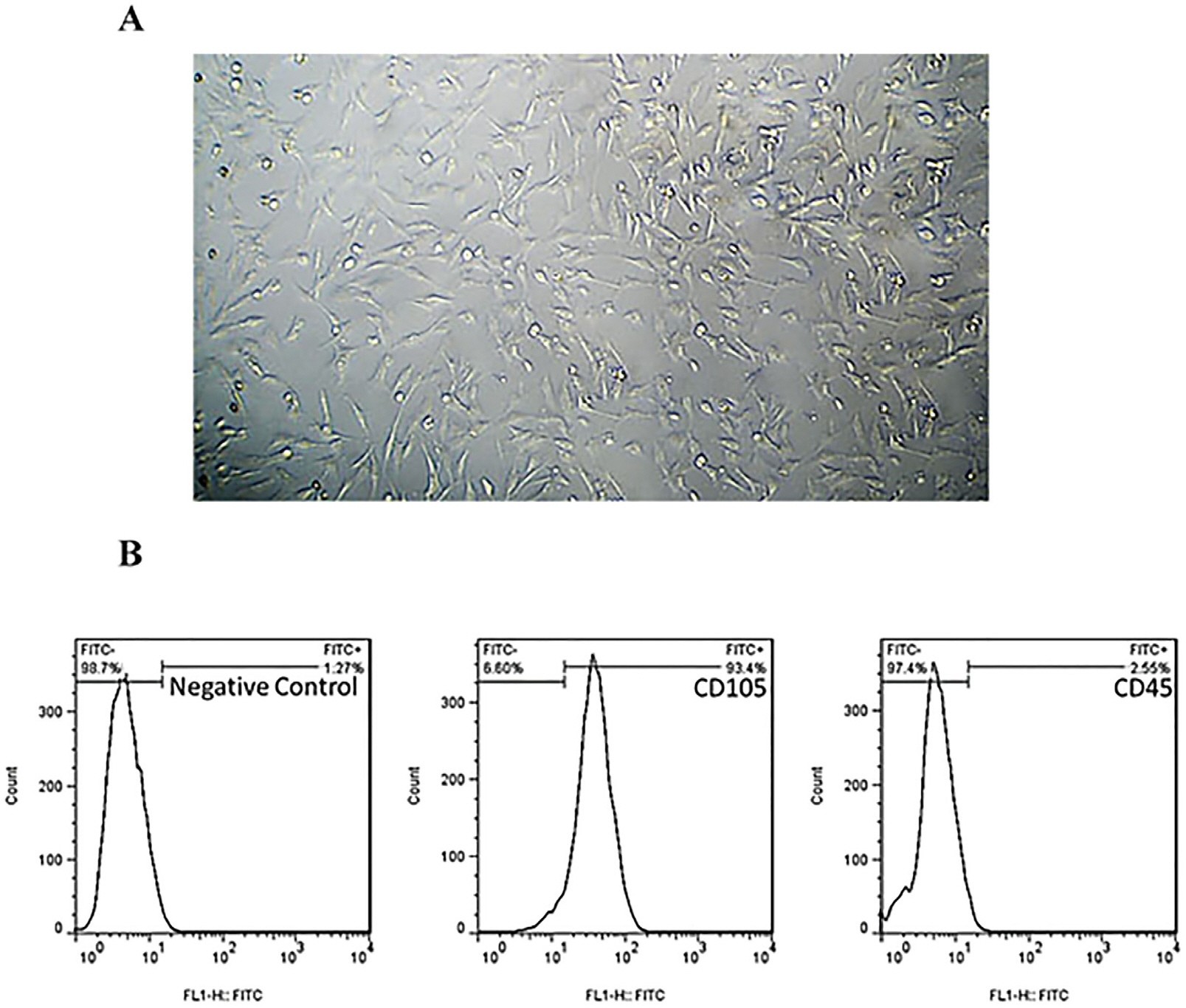
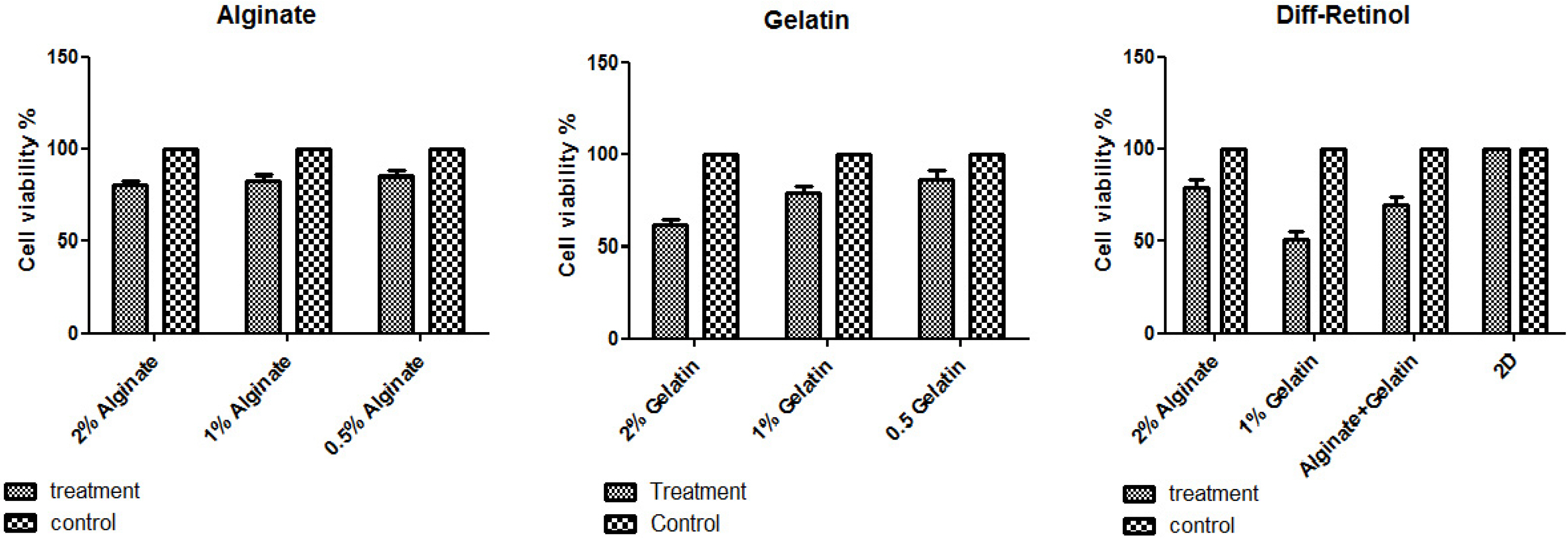
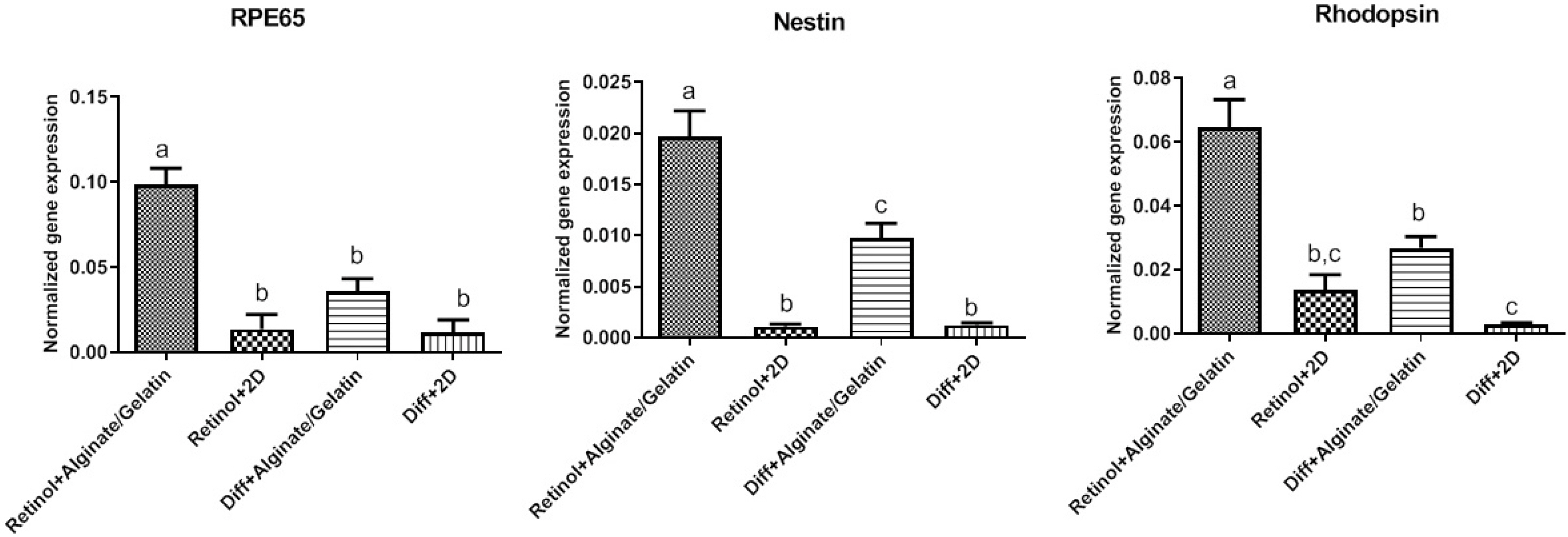
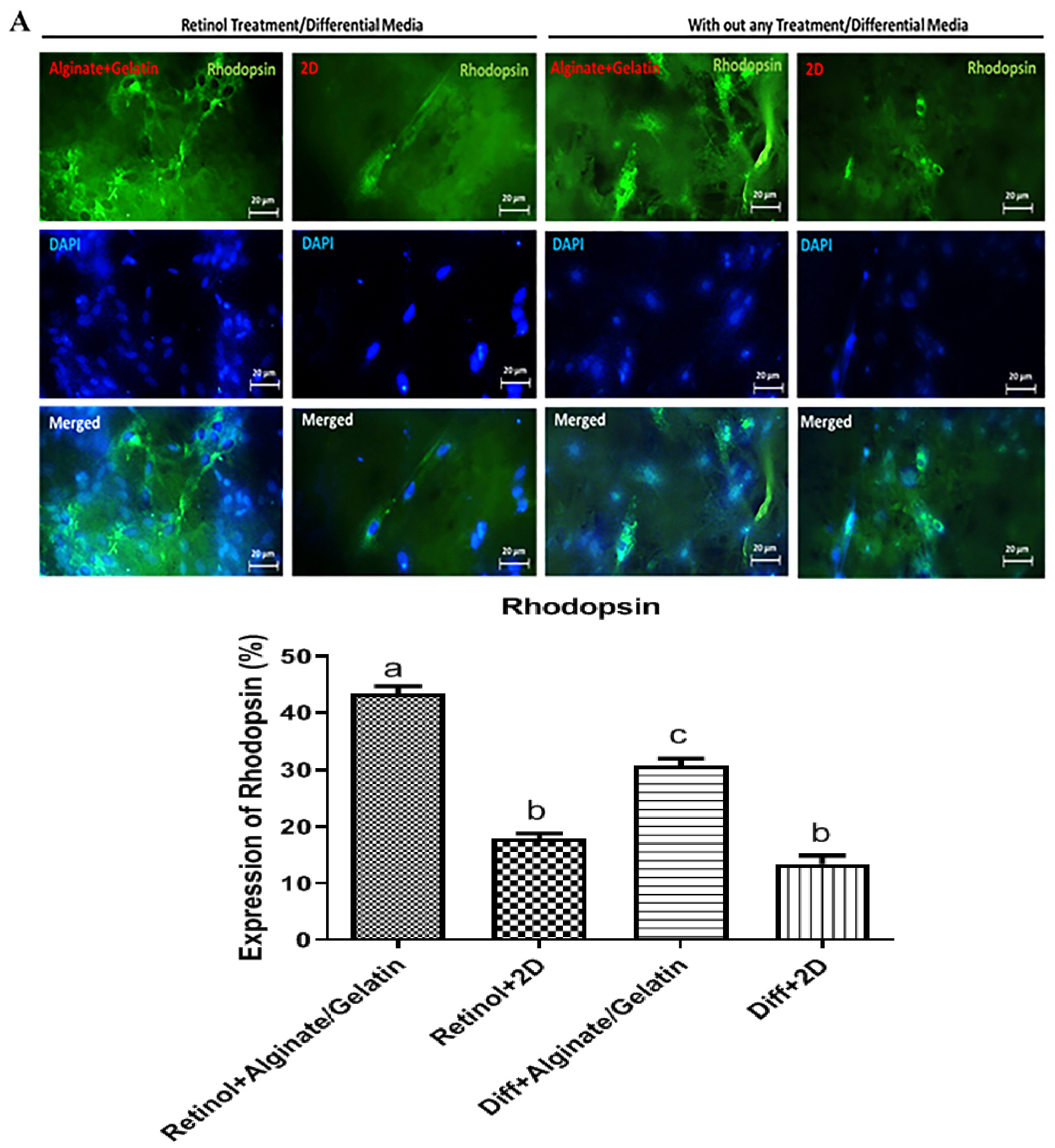
MSCs were extracted from mouse ciliary epithelium, and their identity was verified by detecting specific surface antigens. To provide a three-dimensional in vitro culture system, 2% alginate, 0.5% gelatin and the mixed alginate-gelatin hydrogels were fabricated and checked by SEM. Retinol treatment was performed on MSCs expanded on alginate/gelatin hydrogels and the survival rate and the ability of MSCs to differentiate were examined through measuring expression alterations of retina-specific genes by ICC and qPCR. The cell population isolated from ciliary epithelium contained more than 93.4% cells positive for MSC-specific marker CD105. Alginate/gelatin scaffolds showed to provide an acceptable viability (over 70%) for MSC cultures. Retinol treatment could induce a high expression of rhodopsin protein in MSCs expanded in alginate and alginate-gelatin mixtures. An elevated presentation of Nestin, RPE65 and Rhodopsin genes was detected in retinol-treated cultures expanded on alginate and alginate-gelatin scaffolds.
Conclusions
The results presented here elucidate that retinol treatment of MSCs grown on alginate scaffolds would promote the mouse ciliary epithelium-derived MSCs to differentiate towards retinal neurons.
Figure
Reference
-
References
1. Ramachandra Rao S, Fliesler SJ. 2021; Cholesterol homeostasis in the vertebrate retina: biology and pathobiology. J Lipid Res. 62:100057. DOI: 10.1194/jlr.TR120000979. PMID: 33662384. PMCID: PMC8010701.
Article2. Kha CX, Guerin DJ, Tseng KA. 2020; Studying in vivo retinal progenitor cell proliferation in Xenopus laevis. Methods Mol Biol. 2092:19–33. DOI: 10.1007/978-1-0716-0175-4_2. PMID: 31786778.
Article3. Angileri KM, Gross JM. 2020; dnmt1 function is required to maintain retinal stem cells within the ciliary marginal zone of the zebrafish eye. Sci Rep. 10:11293. DOI: 10.1038/s41598-020-68016-z. PMID: 32647199. PMCID: PMC7347529.
Article4. Centanin L, Hoeckendorf B, Wittbrodt J. 2011; Fate restriction and multipotency in retinal stem cells. Cell Stem Cell. 9:553–562. DOI: 10.1016/j.stem.2011.11.004. PMID: 22136930.
Article5. Tang X, Gao J, Jia X, Zhao W, Zhang Y, Pan W, He J. 2017; Bipotent progenitors as embryonic origin of retinal stem cells. J Cell Biol. 216:1833–1847. DOI: 10.1083/jcb.201611057. PMID: 28465291. PMCID: PMC5461025.
Article6. Eymann J, Salomies L, Macrì S, Di-Poï N. 2019; Variations in the proliferative activity of the peripheral retina correlate with postnatal ocular growth in squamate reptiles. J Comp Neurol. 527:2356–2370. DOI: 10.1002/cne.24677. PMID: 30860599. PMCID: PMC6766921.
Article7. Jorstad NL, Wilken MS, Todd L, Finkbeiner C, Nakamura P, Radulovich N, Hooper MJ, Chitsazan A, Wilkerson BA, Rieke F, Reh TA. 2020; STAT signaling modifies Ascl1 chromatin binding and limits neural regeneration from muller glia in adult mouse retina. Cell Rep. 30:2195–2208.e5. DOI: 10.1016/j.celrep.2020.01.075. PMID: 32075759. PMCID: PMC7148114.
Article8. Aladdad AM, Kador KE. 2019; Adult stem cells, tools for repairing the retina. Curr Ophthalmol Rep. 7:21–29. DOI: 10.1007/s40135-019-00195-z. PMID: 31667009. PMCID: PMC6821446.
Article9. Bammidi S, Bali P, Kalra J, Anand A. 2020; Transplantation efficacy of human ciliary epithelium cells from fetal eye and Lin-ve stem cells from umbilical cord blood in the murine retinal degeneration model of laser injury. Cell Transplant. 29:963689720946031. DOI: 10.1177/0963689720946031. PMID: 33023312. PMCID: PMC7784603. PMID: 4cd4e2ede4b440319cd78077957b6abf.
Article10. Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. 2000; Retinal stem cells in the adult mammalian eye. Science. 287:2032–2036. DOI: 10.1126/science.287.5460.2032. PMID: 10720333.
Article11. Ballios BG, Clarke L, Coles BL, Shoichet MS, Van Der Kooy D. 2012; The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol Open. 1:237–246. DOI: 10.1242/bio.2012027. PMID: 23213414. PMCID: PMC3507281.
Article12. Canola K, Angénieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, Schorderet DF, Arsenijevic Y. 2007; Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 48:446–454. DOI: 10.1167/iovs.06-0190. PMID: 17197566. PMCID: PMC2823590.
Article13. MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. 2006; Retinal repair by transplantation of photoreceptor precursors. Nature. 444:203–207. DOI: 10.1038/nature05161. PMID: 17093405.
Article14. Inoue T, Coles BL, Dorval K, Bremner R, Bessho Y, Kageyama R, Hino S, Matsuoka M, Craft CM, McInnes RR, Tremblay F, Prusky GT, van der Kooy D. 2010; Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells. 28:489–500. DOI: 10.1002/stem.279. PMID: 20014120. PMCID: PMC2933833.
Article15. Huang L, Chen M, Zhang W, Sun X, Liu B, Ge J. 2018; Retinoid acid and taurine promote NeuroD1-induced differentiation of induced pluripotent stem cells into retinal ganglion cells. Mol Cell Biochem. 438:67–76. DOI: 10.1007/s11010-017-3114-x. PMID: 28766169.
Article16. Merhi-Soussi F, Angénieux B, Canola K, Kostic C, Tekaya M, Hornfeld D, Arsenijevic Y. 2006; High yield of cells committed to the photoreceptor fate from expanded mouse retinal stem cells. Stem Cells. 24:2060–2070. DOI: 10.1634/stemcells.2005-0311. PMID: 16644923.
Article17. Macheleidt J, Mattern DJ, Fischer J, Netzker T, Weber J, Schroeckh V, Valiante V, Brakhage AA. 2016; Regulation and role of fungal secondary metabolites. Annu Rev Genet. 50:371–392. DOI: 10.1146/annurev-genet-120215-035203. PMID: 27732794.
Article18. Gudas LJ, Wagner JA. 2011; Retinoids regulate stem cell diffe-rentiation. J Cell Physiol. 226:322–330. DOI: 10.1002/jcp.22417. PMID: 20836077. PMCID: PMC3315372.
Article19. Lee J, Cuddihy MJ, Kotov NA. 2008; Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 14:61–86. DOI: 10.1089/teb.2007.0150. PMID: 18454635.
Article20. Campuzano S, Pelling AE. 2019; Scaffolds for 3D cell culture and cellular agriculture applications derived from non-animal sources. Front Sustain Food Syst. 3:38. DOI: 10.3389/fsufs.2019.00038.
Article21. Pan T, Song W, Cao X, Wang Y. 2016; 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: influence of crosslinking degree and pore architecture on physicochemical properties. J Mater Sci Technol. 32:889–900. DOI: 10.1016/j.jmst.2016.01.007.
Article22. Farokhi M, Shariatzadeh FJ, Solouk A, Mirzadeh H. 2020; Alginate based scaffolds for cartilage tissue engineering: a review. Int J Polym Mater Polym Biomater. 69:230–247. DOI: 10.1080/00914037.2018.1562924.
Article23. Zarif ME. 2018; A review of chitosan-, alginate-, and gelatin-based biocomposites for bone tissue engineering. Biomater Tissue Eng Bull. 5:97–109. DOI: 10.33263/BTEB534.097109.
Article24. Geiger P, Barben M, Grimm C, Samardzija M. 2015; Blue light-induced retinal lesions, intraretinal vascular leakage and edema formation in the all-cone mouse retina. Cell Death Dis. 6:e1985. DOI: 10.1038/cddis.2015.333. PMID: 26583326. PMCID: PMC4670937.
Article25. Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. 2009; Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 4:e8152. DOI: 10.1371/journal.pone.0008152. PMID: 19997644. PMCID: PMC2780911.
Article26. Haghighat M, Iranbakhsh A, Baharara J, Ebadi M, Sotoodehnejadnematalahi F. 2021; Effect of β-carotene on the differentiation potential of ciliary epithelium-derived MSCs isolated from mouse eyes on alginate-based scaffolds. Exp Eye Res. 202:108346. DOI: 10.1016/j.exer.2020.108346. PMID: 33147471.
Article27. Yu JJ, Azzam DB, Chwa M, Schneider K, Cho JH, Hsiang C, Klassen H, Kenney MC, Yang J. 2021; Age-related macular degeneration (AMD) transmitochondrial cybrids protected from cellular damage and death by human retinal progenitor cells (hRPCs). Stem Cells Int. 2021:6655372. DOI: 10.1155/2021/6655372. PMID: 33628267. PMCID: PMC7886532.
Article28. Del Debbio CB, Peng X, Xiong H, Ahmad I. 2013; Adult ciliary epithelial stem cells generate functional neurons and differentiate into both early and late born retinal neurons under non-cell autonomous influences. BMC Neurosci. 14:130. DOI: 10.1186/1471-2202-14-130. PMID: 24148749. PMCID: PMC3856605.
Article29. Yamamoto N, Hiramatsu N, Ohkuma M, Hatsusaka N, Takeda S, Nagai N, Miyachi EI, Kondo M, Imaizumi K, Horiguchi M, Kubo E, Sasaki H. 2021; Novel technique for retinal nerve cell regeneration with electrophysiological functions using human iris-derived iPS cells. Cells. 10:743. DOI: 10.3390/cells10040743. PMID: 33800535. PMCID: PMC8067101. PMID: c5d2afde192941639ab9404484a0bf80.
Article30. Notara M, Lentzsch A, Coroneo M, Cursiefen C. 2018; The role of limbal epithelial stem cells in regulating corneal (Lymph)angiogenic privilege and the micromilieu of the limbal niche following UV exposure. Stem Cells Int. 2018:8620172. DOI: 10.1155/2018/8620172. PMID: 29853920. PMCID: PMC5964490.
Article31. Akrami H, Soheili ZS, Khalooghi K, Ahmadieh H, Rezaie-Kanavi M, Samiei S, Davari M, Ghaderi S, Sanie-Jahromi F. 2009; Retinal pigment epithelium culture;a potential source of retinal stem cells. J Ophthalmic Vis Res. 4:134–141. PMID: 23198062. PMCID: PMC3498558.32. Fischer AJ, Reh TA. 2001; Transdifferentiation of pigmented epithelial cells: a source of retinal stem cells? Dev Neurosci. 23:268–276. DOI: 10.1159/000048710. PMID: 11756742.
Article33. Langhe R, Pearson RA. 2020; Rebuilding the retina: prospects for Müller glial-mediated self-repair. Curr Eye Res. 45:349–360. DOI: 10.1080/02713683.2019.1669665. PMID: 31557060.
Article34. Khoo HE, Ng HS, Yap WS, Goh HJH, Yim HS. 2019; Nutrients for prevention of macular degeneration and eye-related diseases. Antioxidants (Basel). 8:85. DOI: 10.3390/antiox8040085. PMID: 30986936. PMCID: PMC6523787.
Article35. Palczewski K. 2010; Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 31:284–295. DOI: 10.1016/j.tips.2010.03.001. PMID: 20435355. PMCID: PMC2882531.
Article36. Coles BL, Angénieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. 2004; Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A. 101:15772–15777. DOI: 10.1073/pnas.0401596101. PMID: 15505221. PMCID: PMC524825.
Article37. Xu H, Sta Iglesia DD, Kielczewski JL, Valenta DF, Pease ME, Zack DJ, Quigley HA. 2007; Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 48:1674–1682. DOI: 10.1167/iovs.06-1034. PMID: 17389499.
Article38. Reh TA, Levine EM. 1998; Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 36:206–220. DOI: 10.1002/(SICI)1097-4695(199808)36:2<206::AID-NEU8>3.0.CO;2-5. PMID: 9712305.
Article39. Bertolotti E, Neri A, Camparini M, Macaluso C, Marigo V. 2014; Stem cells as source for retinal pigment epithelium transplantation. Prog Retin Eye Res. 42:130–144. DOI: 10.1016/j.preteyeres.2014.06.002. PMID: 24933042.
Article40. Bi Y, Gong M, Zhang X, Zhang X, Jiang W, Zhang Y, Chen J, Liu Y, He TC, Li T. 2010; Pre-activation of retinoid signaling facilitates neuronal differentiation of mesenchymal stem cells. Dev Growth Differ. 52:419–431. DOI: 10.1111/j.1440-169X.2010.01182.x. PMID: 20507357.
Article41. Fawzy El-Sayed KM, Hein D, Dörfer CE. 2019; Retinol/inflammation affect stemness and differentiation potential of gingival stem/progenitor cells via Wnt/β-catenin. J Perio-dontal Res. 54:413–423. DOI: 10.1111/jre.12643. PMID: 30830694.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Substrates and Hydrostatic Pressure on Differentiation of Mesenchymal Stem Cell
- Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells
- Adult Mesenchymal Stem Cells for Cell Therapy in Clinical Application
- Mesenchymal Stem Cells for Tissue Engineering and Regenerative Medicine
- Biomimetic Polymer Scaffolds to Promote Stem Cell-Mediated Osteogenesis