Intest Res.
2022 Apr;20(2):269-273. 10.5217/ir.2021.00064.
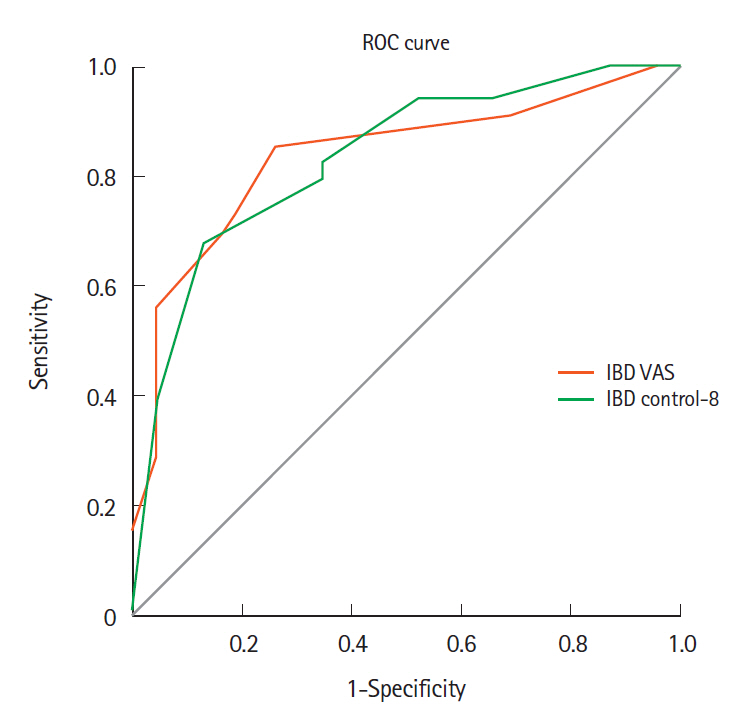
Correlation of fecal calprotectin and patient-reported outcome measures in patients with ulcerative colitis
- Affiliations
-
- 1Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2529573
- DOI: http://doi.org/10.5217/ir.2021.00064
Figure
Cited by 1 articles
-
A novel serum biomarker of endoscopic mucosal healing in inflammatory bowel disease
Hyoun Woo Kang
Intest Res. 2024;22(1):3-4. doi: 10.5217/ir.2023.00198.
Reference
-
1. Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006; 4:1491–1501.
Article2. Burke LB, Kennedy DL, Miskala PH, Papadopoulos EJ, Trentacosti AM. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther. 2008; 84:281–283.
Article3. Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014; 12:1246–1256.
Article4. Bodger K, Ormerod C, Shackcloth D, Harrison M; IBD Control Collaborative. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-control questionnaire. Gut. 2014; 63:1092–1102.
Article5. Kim DJ, Jeoun YM, Lee DW, Koo JS, Lee SW. Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis. Intest Res. 2018; 16:563–570.
Article6. Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005; 54:782–788.
Article7. Dudeja P, Bahuguna P, Singh A, Bhatnagar N. Refining a socioeconomic status scale for use in community-based health research in India. J Postgrad Med. 2015; 61:77–83.
Article8. Keller R, Mazurak N, Fantasia L, et al. Quality of life in inflammatory bowel diseases: it is not all about the bowel. Intest Res. 2021; 19:45–52.
Article9. Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012; 12:108.
Article10. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD). Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article11. Dulai PS, Battat R, Barsky M, et al. Incorporating fecal calprotectin into clinical practice for patients with moderate-to-severely active ulcerative colitis treated with biologics or smallmolecule inhibitors. Am J Gastroenterol. 2020; 115:885–894.
Article12. Bertani L, Blandizzi C, Mumolo MG, et al. Fecal calprotectin predicts mucosal healing in patients with ulcerative colitis treated with biological therapies: a prospective study. Clin Transl Gastroenterol. 2020; 11:e00174.
Article13. Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016; 150:96–102.
Article14. Lobatón T, Rodríguez-Moranta F, Lopez A, Sánchez E, Rodríguez-Alonso L, Guardiola J. A new rapid quantitative test for fecal calprotectin predicts endoscopic activity in ulcerative colitis. Inflamm Bowel Dis. 2013; 19:1034–1042.
Article15. Walsh A, Kormilitzin A, Hinds C, et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis: a prospective analysis. J Crohns Colitis. 2019; 13:424–430.
Article16. Samaan M, Cunningham G, Tamilarasan G, et al. P143 Validation of the two-item UC PRO2 against SCCAI, biochemical disease activity and quality of life. Gut. 2021; 70(Suppl 1):A116–A117.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Fecal Calprotectin in Inflammatory Bowel Disease
- Fecal Immunochemical Test and Fecal Calprotectin Measurement Are Noninvasive Monitoring Tools for Predicting Endoscopic Activity in Patients with Ulcerative Colitis
- Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring