Cancer Res Treat.
2022 Apr;54(2):579-589. 10.4143/crt.2021.496.
Clinicopathologic Characteristics and Clinical Outcome of Localized Liposarcoma: A Single-Center Experience over 25 Years and Evaluation of PD-L1 Expression
- Affiliations
-
- 1Department of Oncology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea
- 2Center for Breast Cancer, National Cancer Center Korea, Goyang, Korea
- 3Department of Orthopedic Surgery, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Radiation Oncology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Radiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Pathology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2528227
- DOI: http://doi.org/10.4143/crt.2021.496
Abstract
- Purpose
For liposarcoma (LPS), clinical course and proper treatment strategies have not been well-established. Recently, immune-checkpoint inhibitors have shown potential efficacy in LPS. We aimed to describe the clinical course of LPS and evaluate the clinical impact of programmed death-ligand 1 (PD-L1).
Materials and Methods
We reviewed all consecutive patients (n=332) who underwent curative-intent surgery for localized LPS at Asan Medical Center between 1989 and 2017. PD-L1 testing was performed in well-differentiated and dedifferentiated LPS.
Results
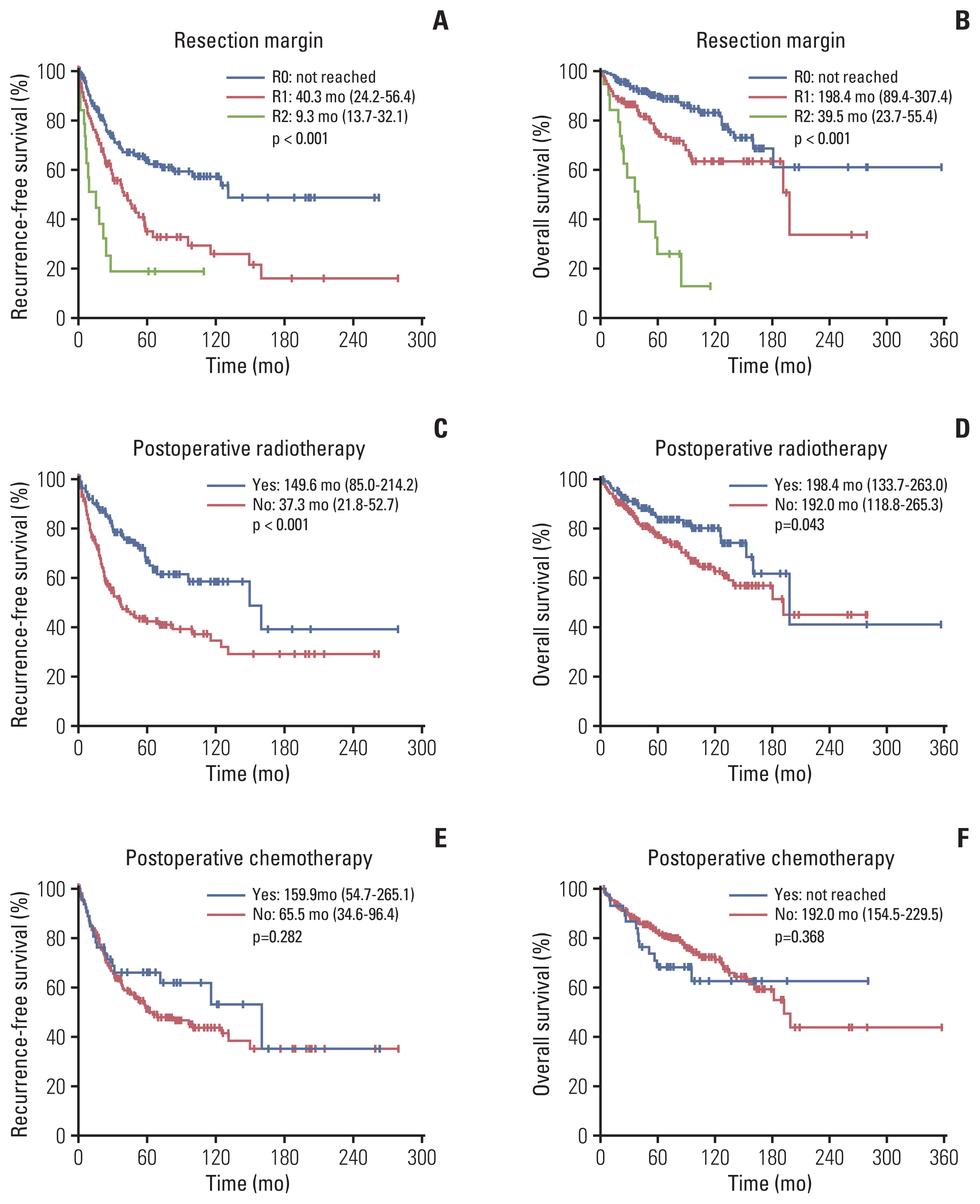
The median age was 56 years with males comprising 60.8%. Abdomen-pelvis (47.6%) and well-differentiated (37.7%) were the most frequent primary site and histologic subtype, respectively. During a median follow-up of 81.2 months, recurrence was observed in 135 (40.7%), and 86.7% (117/135) were loco-regional. Well-differentiated subtype (hazard ratio [HR], 0.38), abdomen-pelvis origin (HR, 2.43), tumor size larger than 5 cm (HR, 1.83), positive resection margin (HR, 2.58), and postoperative radiotherapy (HR, 0.36) were significantly related with recurrence-free survival as well as visceral involvement (HR, 1.84) and multifocality (HR, 3.79) in abdomen-pelvis LPS. PD-L1 was positive in 31.5% (23/73) and 51.3% (39/76) of well-differentiated and dedifferentiated LPS, respectively, but had no impact on survival outcomes.
Conclusion
Clinical course of LPS was heterogeneous according to histology and anatomic location. Clear resection margin was important to lower recurrence and postoperative radiotherapy might have additional benefit. A decent portion of well-differentiated and dedifferentiated LPS were positive for PD-L1, but its prognostic role was unclear. Further research is needed to determine clinical implications of PD-L1, especially for advanced-stage LPS with unmet needs for effective systemic treatment.
Keyword
Figure
Reference
-
References
1. Ducimetiere F, Lurkin A, Ranchere-Vince D, Decouvelaere AV, Peoc’h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011; 6:e20294.
Article2. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014; 46:95–104.
Article3. Kaushal A, Citrin D. The role of radiation therapy in the management of sarcomas. Surg Clin North Am. 2008; 88:629–46.
Article4. Chung PW, Deheshi BM, Ferguson PC, Wunder JS, Griffin AM, Catton CN, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009; 115:3254–61.
Article5. Katz D, Boonsirikamchai P, Choi H, Lazar AJ, Wang WL, Xiao L, et al. Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res. 2012; 2:2.
Article6. Marshall S, Nakano K, Sugiura Y, Taira S, Ono M, Tomomatsu J, et al. Outcome for advanced or metastatic soft tissue sarcoma of nonextremities treated with doxorubicin-based chemotherapy: a retrospective study from a single cancer institution. Sarcoma. 2018; 2018:8926598.
Article7. National Comprehensive Cancer Network. Soft tissue sarcoma (version 6. 2019) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Feb 10]. Available from: https://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf .8. Jones RL, Fisher C, Al-Muderis O, Judson IR. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005; 41:2853–60.
Article9. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018; 19:416–26.
Article10. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017; 18:1493–501.
Article11. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017; 3:e172411.12. Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008; 97:298–313.
Article13. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006; 244:381–91.
Article14. Knebel C, Lenze U, Pohlig F, Lenze F, Harrasser N, Suren C, et al. Prognostic factors and outcome of liposarcoma patients: a retrospective evaluation over 15 years. BMC Cancer. 2017; 17:410.15. Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003; 238:358–70.
Article16. Anaya DA, Lahat G, Liu J, Xing Y, Cormier JN, Pisters PW, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009; 249:137–42.17. Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin KH, et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016; 16:434.
Article18. D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015; 46:357–65.
Article19. Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013; 8:e82870.
Article20. Paydas S, Bagir EK, Deveci MA, Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016; 33:93.
Article21. Orth MF, Buecklein VL, Kampmann E, Subklewe M, Noessner E, Cidre-Aranaz F, et al. Expression patterns of PD-L1 and PD-1 provide rationales for immune checkpoint inhibition in soft tissue sarcomas. Preprint at http://doi.org/10.1101/569418(2019) .
Article22. Yan L, Wang Z, Cui C, Guan X, Dong B, Zhao M, et al. Comprehensive immune characterization and T-cell receptor repertoire heterogeneity of retroperitoneal liposarcoma. Cancer Sci. 2019; 110:3038–48.
Article23. Zhu Z, Jin Z, Zhang M, Tang Y, Yang G, Yuan X, et al. Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget. 2017; 8:59570–80.
Article24. Torabi A, Amaya CN, Wians FH Jr, Bryan BA. PD-1 and PDL1 expression in bone and soft tissue sarcomas. Pathology. 2017; 49:506–13.
Article25. Tseng WW, Malu S, Zhang M, Chen J, Sim GC, Wei W, et al. Analysis of the intratumoral adaptive immune response in well differentiated and dedifferentiated retroperitoneal liposarcoma. Sarcoma. 2015; 2015:547460.
Article26. Keung EZ, Lazar AJ, Torres KE, Wang WL, Cormier JN, Ashleigh Guadagnolo B, et al. Phase II study of neoadjuvant checkpoint blockade in patients with surgically resectable undifferentiated pleomorphic sarcoma and dedifferentiated liposarcoma. BMC Cancer. 2018; 18:913.
Article27. Keung EZ, Burgess M, Salazar R, Parra ER, Rodrigues-Canales J, Bolejack V, et al. Correlative analyses of the SARC028 trial reveal an association between sarcoma-associated immune infiltrate and response to pembrolizumab. Clin Cancer Res. 2020; 26:1258–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- Temporal evolution of programmed death-ligand 1 expression in patients with non-small cell lung cancer