Cancer Res Treat.
2022 Apr;54(2):525-540. 10.4143/crt.2021.373.
A Hypoxia-Induced SCFFBXL1 E3 Ligase Ubiquitinates and Degrades the MEN1 Tumor Suppressor to Promote Colorectal Cancer Tumorigenesis
- Affiliations
-
- 1Department of Gastroenterology, Jiangxi Provincial People's Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, China
- 2Department of Oncology, Jiangxi Provincial People's Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, China
- KMID: 2528222
- DOI: http://doi.org/10.4143/crt.2021.373
Abstract
- Purpose
Emerging evidence has shown that SKP1-cullin-1-F-box-protein (SCF) E3 ligases contribute to the pathogenesis of different cancers by mediating the ubiquitination and degradation of tumor suppressors. However, the functions of SCF E3 ligases in the pathogenesis of colorectal cancer (CRC) remain obscure.
Materials and Methods
The cancerous and adjacent noncancerous tissues from CRC patients were collected, and protein levels were analyzed. Lentiviral short hairpin RNA (shRNA) and plasmid transfection were used to knock down and overexpress gene expression in CRC cell lines. Immunoprecipitation (IP), mass spectrometry, and co-IP analyses were used to determine protein interactions and the assembly of the SCF complex. Cell proliferation, migration, and tumor xenograft assays were performed to examine the effects of SCF members on CRC cell growth in vitro and in vivo.
Results
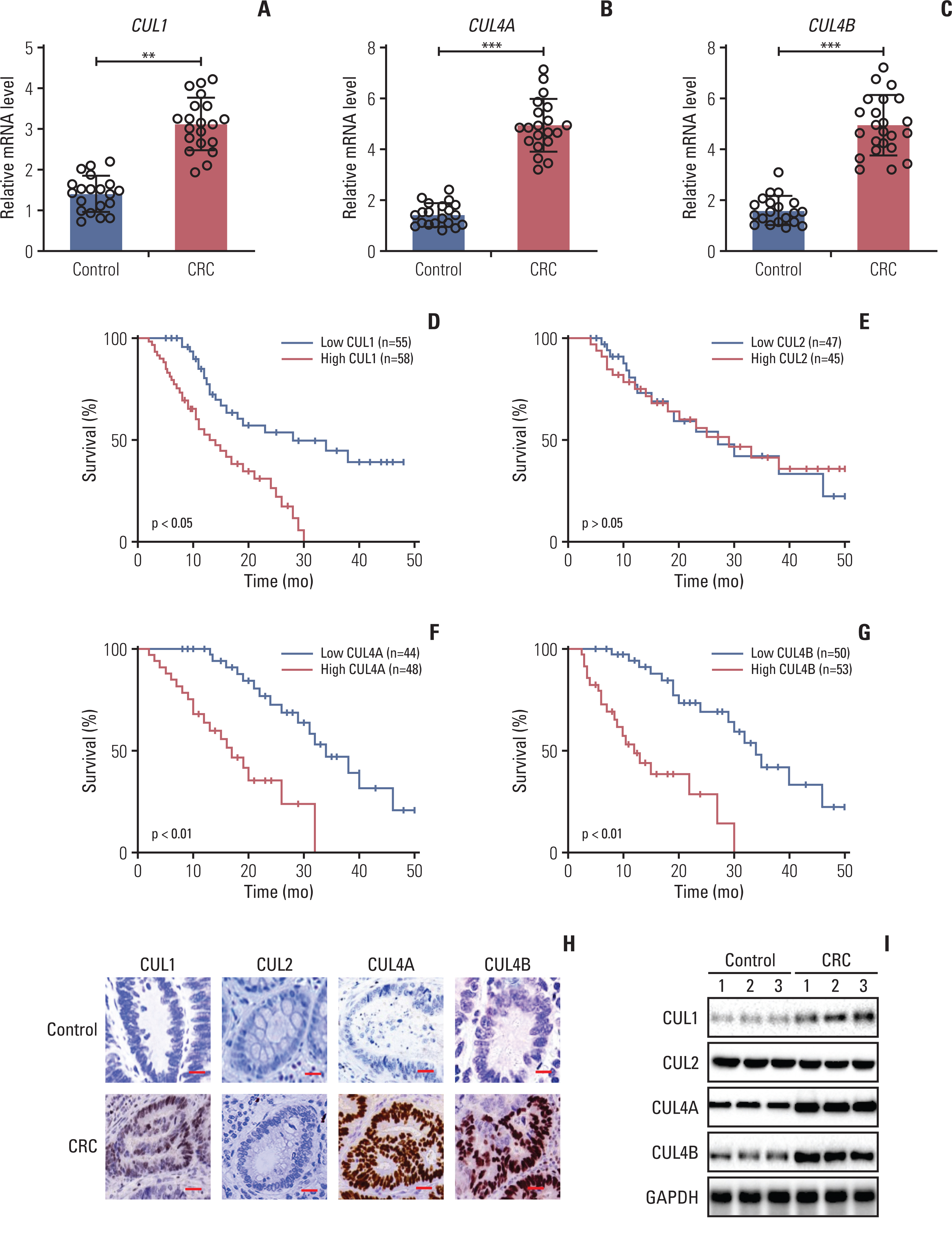
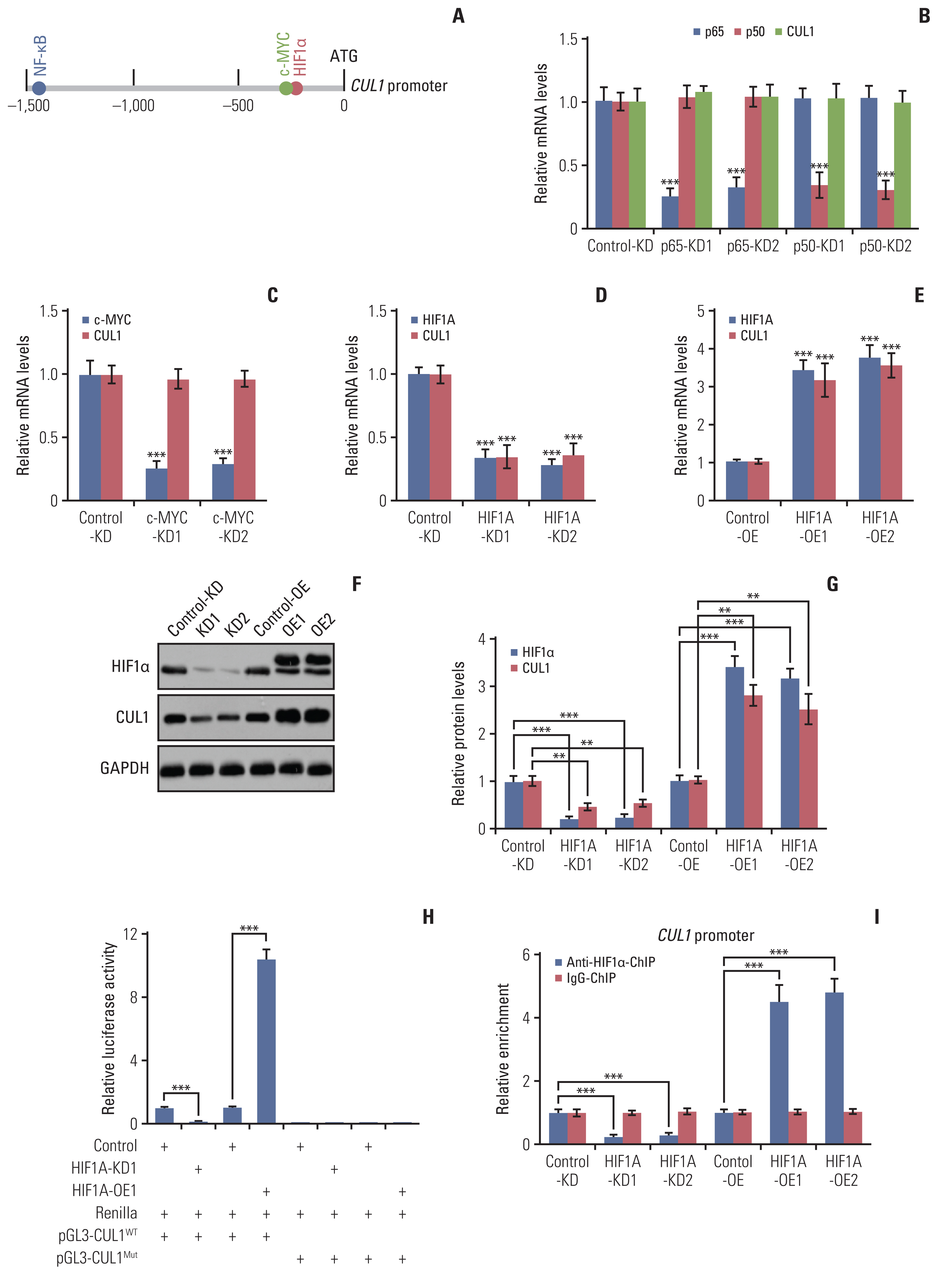
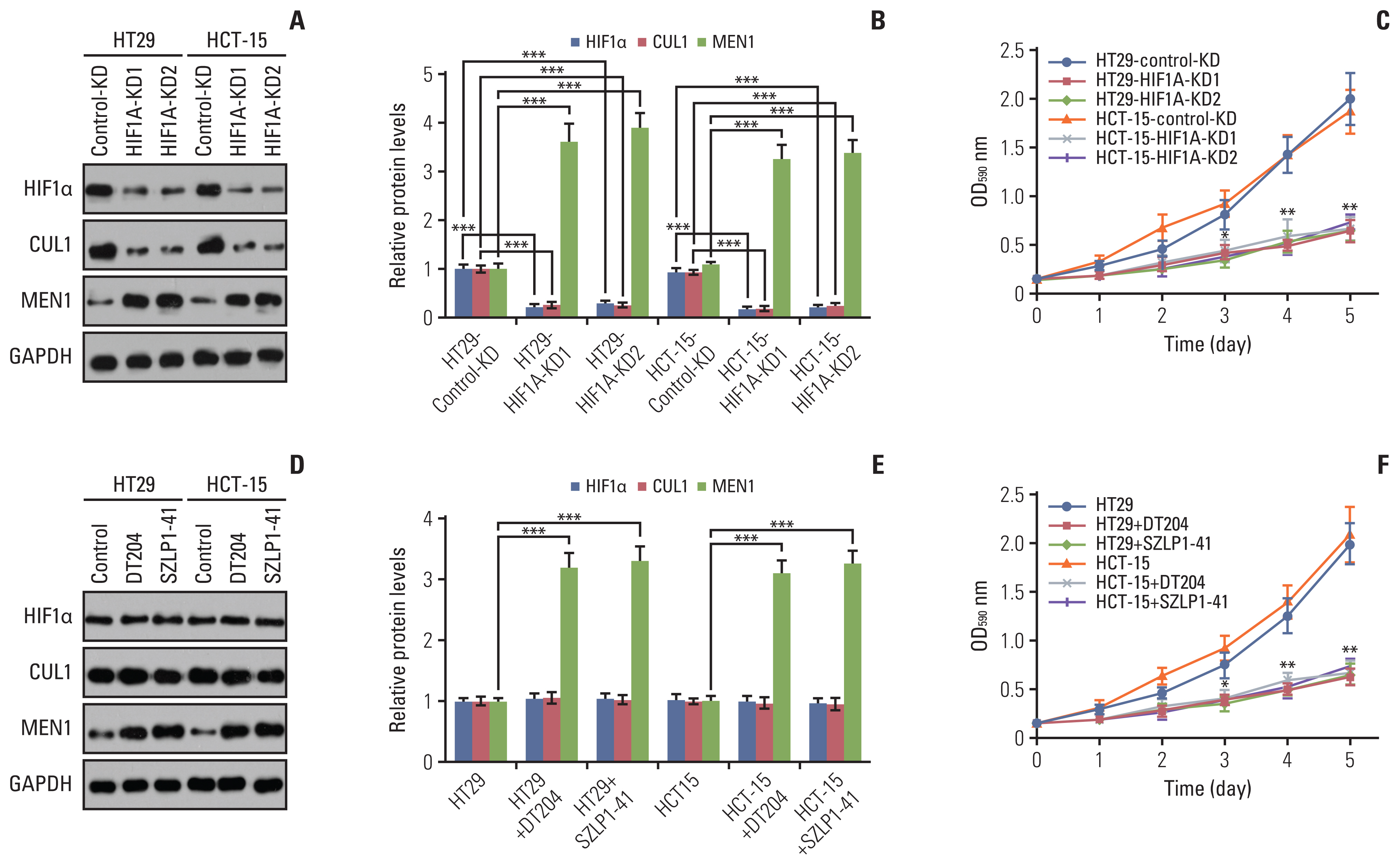
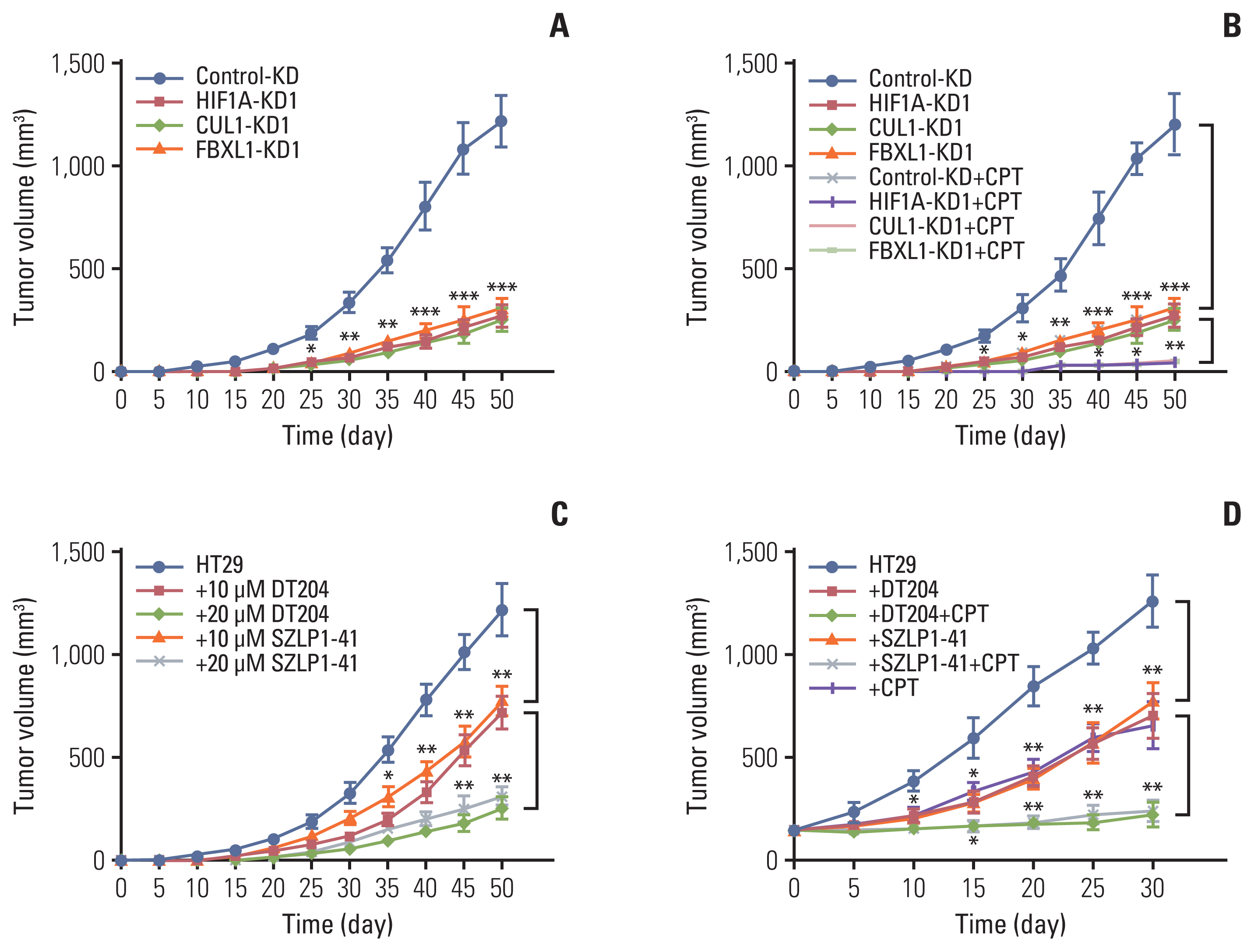
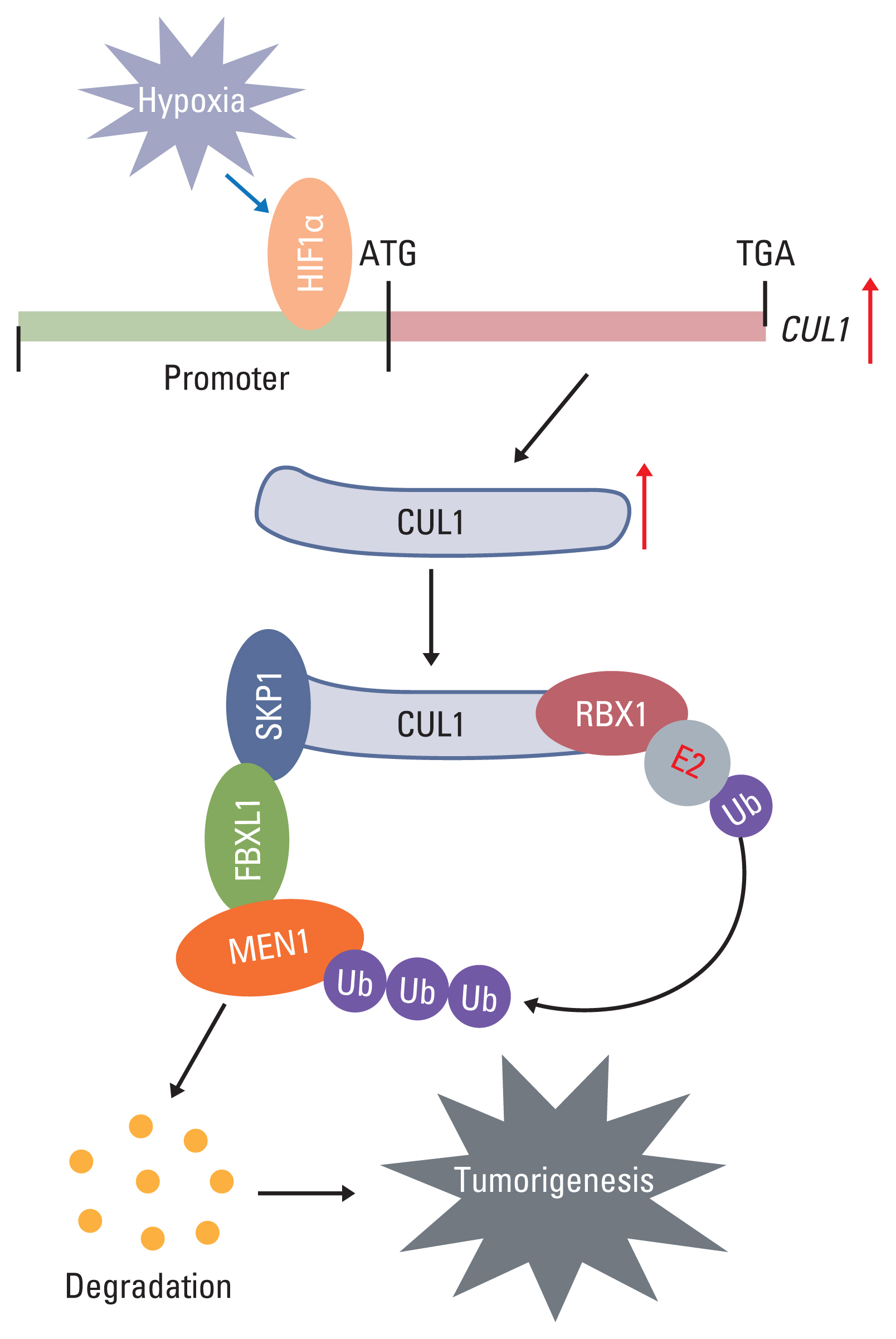
Hypoxia activated the docking of hypoxia-inducible factor 1α (HIF1α) onto the CUL1 promoter and induced CUL1 expression in CRC cells. CUL1 coupled with RBX1, SKP1, and FBXL1 to assemble the SCFFBXL1 complex in CRC biopsies and cells. The SCFFBXL1 E3 ligase specifically ubiquitinated and degraded the MEN1 tumor suppressor. Knockdown of HIF1α or SCFFBXL1 members, or blockage of SCFFBXL1 by two inhibitors (DT204 and SZLP1-41) caused the accumulation of MEN1 protein and led to a significant decrease in cell proliferation and migration in vitro and tumor growth in vivo.
Conclusion
The SCFFBXL1 E3 ligase is required for the ubiquitination of MEN1, and disruption of this complex may represent a new therapeutic strategy for the treatment of CRC.
Keyword
Figure
Reference
-
References
1. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019; 14:89–103.
Article2. Karimi S, Abdi A, Khatony A, Akbari M, Faraji A. Epidemiology of colorectal cancer and the risk factors in Kermanshah province-Iran 2009–2014. J Gastrointest Cancer. 2019; 50:740–3.
Article3. Yao N, Wang J, Cai Y, Yuan J, Wang H, Gong J, et al. Patterns of cancer screening, incidence and treatment disparities in China: protocol for a population-based study. BMJ Open. 2016; 6:e012028.
Article4. Huang Q, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis. 2010; 15:1292–311.
Article5. Weathington NM, Mallampalli RK. Emerging therapies targeting the ubiquitin proteasome system in cancer. J Clin Invest. 2014; 124:6–12.
Article6. Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett. 2011; 585:2803–9.
Article7. Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007; 128:141–56.
Article8. Mulder MP, Witting K, Berlin I, Pruneda JN, Wu KP, Chang JG, et al. A cascading activity-based probe sequentially targets E1-E2-E3 ubiquitin enzymes. Nat Chem Biol. 2016; 12:523–30.
Article9. Chen Z, Sui J, Zhang F, Zhang C. Cullin family proteins and tumorigenesis: genetic association and molecular mechanisms. J Cancer. 2015; 6:233–42.
Article10. Zhao Y, Sun Y. Cullin-RING Ligases as attractive anti-cancer targets. Curr Pharm Des. 2013; 19:3215–25.
Article11. Soucy TA, Dick LR, Smith PG, Milhollen MA, Brownell JE. The NEDD8 conjugation pathway and its relevance in cancer biology and therapy. Genes Cancer. 2010; 1:708–16.
Article12. Liu H, Lu W, He H, Wu J, Zhang C, Gong H, et al. Inflammation-dependent overexpression of c-Myc enhances CRL4 (DCAF4) E3 ligase activity and promotes ubiquitination of ST7 in colitis-associated cancer. J Pathol. 2019; 248:464–75.13. Nelakurti DD, Pappula AL, Rajasekaran S, Miles WO, Petreaca RC. Comprehensive analysis of MEN1 mutations and their role in cancer. Cancers (Basel). 2020; 12:2616.
Article14. Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci. 2013; 38:394–402.
Article15. Kamilaris CDC, Stratakis CA. Multiple endocrine neoplasia type 1 (MEN1): an update and the significance of early genetic and clinical diagnosis. Front Endocrinol (Lausanne). 2019; 10:339.
Article16. Li C, Xiao XQ, Qian YH, Zhou ZY. The CtBP1-p300-FOXO3a transcriptional complex represses the expression of the apoptotic regulators Bax and Bim in human osteosarcoma cells. J Cell Physiol. 2019; 234:22365–77.17. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010; 40:294–309.
Article18. Xie J, Jin Y, Wang G. The role of SCF ubiquitin-ligase complex at the beginning of life. Reprod Biol Endocrinol. 2019; 17:101.
Article19. Malek E, Abdel-Malek MA, Jagannathan S, Vad N, Karns R, Jegga AG, et al. Pharmacogenomics and chemical library screens reveal a novel SCF(SKP2) inhibitor that overcomes Bortezomib resistance in multiple myeloma. Leukemia. 2017; 31:645–53.
Article20. Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013; 154:556–68.
Article21. Penas C, Ramachandran V, Ayad NG. The APC/C ubiquitin ligase: from cell biology to tumorigenesis. Front Oncol. 2011; 1:60.
Article22. Galindo-Moreno M, Giraldez S, Limon-Mortes MC, Belmonte-Fernandez A, Reed SI, Saez C, et al. SCF(FBXW7)-mediated degradation of p53 promotes cell recovery after UV-induced DNA damage. FASEB J. 2019; 33:11420–30.
Article23. Lu Y, Li J, Cheng D, Parameswaran B, Zhang S, Jiang Z, et al. The F-box protein FBXO44 mediates BRCA1 ubiquitination and degradation. J Biol Chem. 2012; 287:41014–22.
Article24. Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003; 11:1445–56.25. Chen Z, Wang K, Hou C, Jiang K, Chen B, Chen J, et al. CRL4B(DCAF11) E3 ligase targets p21 for degradation to control cell cycle progression in human osteosarcoma cells. Sci Rep. 2017; 7:1175.
Article26. Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020; 5:90.
Article27. Aubry A, Yu T, Bremner R. Preclinical studies reveal MLN4924 is a promising new retinoblastoma therapy. Cell Death Discov. 2020; 6:2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Ubiquitin-Proteasome System and F-box Proteins in Pathogenic Fungi
- Phospholipase D2 promotes degradation of hypoxia-inducible factor-1alpha independent of lipase activity
- A Study on the Expression of p53 and nm23 Protein in the Colorectal Adenoma and Carcinoma
- Mannose Attenuates Colitis-Associated Colorectal Tumorigenesis by Targeting Tumor-Associated Macrophages
- The Role of MicroRNAs in Colorectal Cancer