Cancer Res Treat.
2022 Apr;54(2):396-405. 10.4143/crt.2021.393.
Influence of Concurrent and Adjuvant Temozolomide on Health-Related Quality of Life of Patients with Grade III Gliomas: A Secondary Analysis of a Randomized Clinical Trial (KNOG-1101 Study)
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Neurosurgery, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 6Department of Radiation Oncology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- 7Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Neurosurgery, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 9Department of Neuro-Oncology Clinic, Center for Specific Organs Cancer, National Cancer Center Hospital, National Cancer Center, Goyang, Korea
- 10Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 11Department of Neurosurgery, Inha University Hospital, Inha University School of Medicine, Incheon, Korea
- 12Division of Cancer Epidemiology and Management, Research Institute, National Cancer Center, Goyang, Korea
- 13Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 14Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 15Department of Radiology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 16Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2528209
- DOI: http://doi.org/10.4143/crt.2021.393
Abstract
- Purpose
The KNOG-1101 study showed improved 2-year PFS with temozolomide during and after radiotherapy compared to radiotherapy alone for patients with anaplastic gliomas. This trial investigates the effect of concurrent and adjuvant temozolomide on health-related quality of life (HRQoL).
Materials and Methods
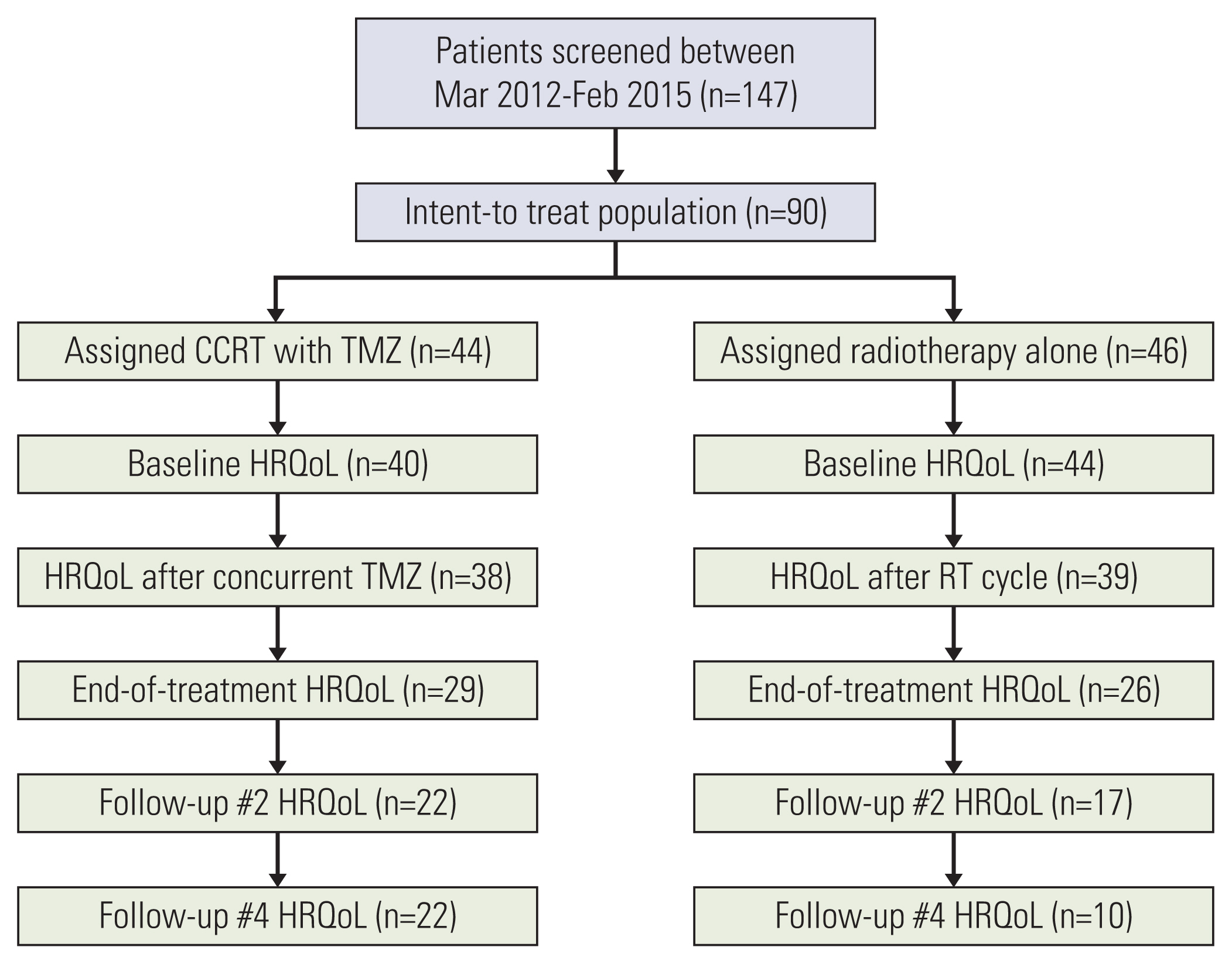
In this randomized, open-label, phase II trial, 90 patients with World Health Organization grade III glioma were enrolled across multiple centers in South Korea between March 2012 to February 2015 and followed up through 2017. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) and 20-item EORTC QLQ-Brain Neoplasm (QLQ-BN20) were used to compare HRQoL between patients assigned to concurrent chemoradiotherapy with temozolomide followed by 6 cycles of adjuvant temozolomide (arm A) and radiotherapy (RT) alone (arm B).
Results
Of the 90 patients in the study, 84 patients (93.3%) completed the baseline HRQoL questionnaire. Emotional functioning, fatigue, nausea and vomiting, dyspnea, constipation, appetite loss, diarrhea, seizures, itchy skin, drowsiness, hair loss, and bladder control were not affected by the addition of temozolomide. All other items did not differ significantly between arm A and arm B throughout treatment. Global health status particularly stayed consistent at the end of adjuvant temozolomide (p=0.47) and at the end of RT (p=0.33).
Conclusion
The addition of concurrent and adjuvant temozolomide did not show negative influence on HRQoL with improvement of progression-free survival for patients with anaplastic gliomas. The absence of systematic and clinically relevant changes in HRQoL suggests that an overall long-term net clinical benefit exists for concurrent and adjuvant temozolomide.
Figure
Reference
-
References
1. Izquierdo C, Joubert B, Ducray F. Anaplastic gliomas in adults: an update. Curr Opin Oncol. 2017; 29:434–42.
Article2. Siker ML, Chakravarti A, Mehta MP. Should concomitant and adjuvant treatment with temozolomide be used as standard therapy in patients with anaplastic glioma? Crit Rev Oncol Hematol. 2006; 60:99–111.
Article3. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352:997–1003.
Article4. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–66.
Article5. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–96.
Article6. van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CAT-NON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017; 390:1645–53.
Article7. Kim YH, Park CK, Cho WH, Kim IA, Moon S, Choe G, et al. Temozolomide during and after radiation therapy for WHO grade III gliomas: preliminary report of a prospective multicenter study. J Neurooncol. 2011; 103:503–12.
Article8. Vogelbaum MA, Hu C, Peereboom DM, Macdonald DR, Giannini C, Suh JH, et al. Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: long term results of RTOG BR0131. J Neurooncol. 2015; 124:413–20.
Article9. Berghoff A, van den Bent M. How I treat anaplastic glioma without 1p/19q codeletion. ESMO Open. 2019; 4:e000534.
Article10. Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998; 90:1473–9.
Article11. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010; 120:707–18.
Article12. Kamiryo T, Tada K, Shiraishi S, Shinojima N, Kochi M, Ushio Y. Correlation between promoter hypermethylation of the O6-methylguanine-deoxyribonucleic acid methyltransferase gene and prognosis in patients with high-grade astrocytic tumors treated with surgery, radiotherapy, and 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea-based chemotherapy. Neurosurgery. 2004; 54:349–57.
Article13. Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000; 18:636–45.
Article14. Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31:337–43.
Article15. van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31:344–50.16. Kim CW, Joo JD, Kim YH, Han JH, Kim CY. Health-related quality of life in brain tumor patients treated with surgery: preliminary result of a single institution. Brain Tumor Res Treat. 2016; 4:87–93.
Article17. Hwang K, Kim TM, Park CK, Chang JH, Jung TY, Kim JH, et al. Concurrent and adjuvant temozolomide for newly diagnosed grade III gliomas without 1p/19q co-deletion: a randomized, open-label, phase 2 study (KNOG-1101 Study). Cancer Res Treat. 2020; 52:505–15.
Article18. Jenkins RB, Curran W, Scott CB, Cairncross G. Pilot evaluation of 1p and 19q deletions in anaplastic oligodendrogliomas collected by a national cooperative cancer treatment group. Am J Clin Oncol. 2001; 24:506–8.
Article19. Walker MD, Alexander E Jr, Hunt WE, MacCarty CS, Mahaley Ms Jr, Mealey J Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg. 1978; 49:333–43.20. Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980; 303:1323–9.
Article21. DeAngelis LM. Brain tumors. N Engl J Med. 2001; 344:114–23.
Article22. Simonetti G, Gaviani P, Innocenti A, Botturi A, Lamperti E, Silvani A. Update on treatment strategies for anaplastic glioma: a review of literature. Neurol Sci. 2014; 35:977–81.
Article23. Sasaki H, Yoshida K. Treatment recommendations for adult patients with diffuse gliomas of grades II and III according to the new WHO classification in 2016. Neurol Med Chir (Tokyo). 2017; 57:658–66.
Article24. Chibbaro S, Benvenuti L, Caprio A, Carnesecchi S, Pulera F, Faggionato F, et al. Temozolomide as first-line agent in treating high-grade gliomas: phase II study. J Neurooncol. 2004; 67:77–81.
Article25. Joo JD, Chang JH, Kim JH, Hong YK, Kim YH, Kim CY. Temozolomide during and after radiotherapy for newly diagnosed glioblastomas: a prospective multicenter study of Korean patients. J Korean Neurosurg Soc. 2012; 52:92–7.
Article26. Joo JD, Kim H, Kim YH, Han JH, Kim CY. Validation of the effectiveness and safety of temozolomide during and after radiotherapy for newly diagnosed glioblastomas: 10-year experience of a single institution. J Korean Med Sci. 2015; 30:1597–603.
Article27. Bae SH, Park MJ, Lee MM, Kim TM, Lee SH, Cho SY, et al. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014; 29:980–4.
Article28. Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJ. Health-related quality of life in high-grade glioma patients. Chin J Cancer. 2014; 33:40–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent and Adjuvant Temozolomide for Newly Diagnosed Grade IIIGliomas without 1p/19q Co-deletion: A Randomized, Open-Label,Phase 2 Study (KNOG-1101 Study)
- Overcoming Treatment Resistance in High Grade Gliomas
- Managing Side Effects of Cytotoxic Chemotherapy in Patients With High Grade Gliomas
- Validation of the Effectiveness and Safety of Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas: 10-year Experience of a Single Institution
- Health-related quality of life and late morbidity in concurrent chemoradiation and radiotherapy alone in patients with locally advanced cervical carcinoma