Nutr Res Pract.
2022 Apr;16(2):173-193. 10.4162/nrp.2022.16.2.173.
Protective effects of Populus tomentiglandulosa against cognitive impairment by regulating oxidative stress in an amyloid beta25–35 -induced Alzheimer's disease mouse model
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busan 46241, Korea
- 2Department of Plant Science and Technology, Chung-Ang University, Anseong 17546, Korea
- 3Natural Product Institute of Science and Technology, Anseong 17546, Korea
- 4Department of Food Science, Gyeongsang National University, Jinju 52725, Korea
- KMID: 2527809
- DOI: http://doi.org/10.4162/nrp.2022.16.2.173
Abstract
- BACKGROUND/OBJECTIVES
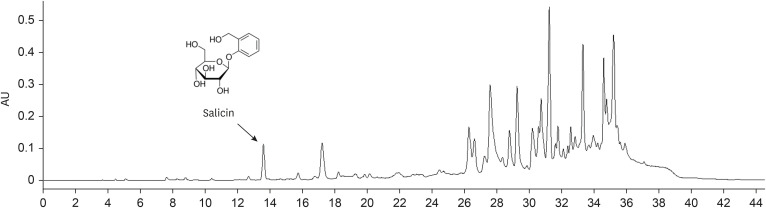
Alzheimer's disease (AD) is one of the most representative neurodegenerative disease mainly caused by the excessive production of amyloid beta (Aβ). Several studies on the antioxidant activity and protective effects of Populus tomentiglandulosa (PT) against cerebral ischemia-induced neuronal damage have been reported. Based on this background, the present study investigated the protective effects of PT against cognitive impairment in AD.
MATERIALS/METHODS
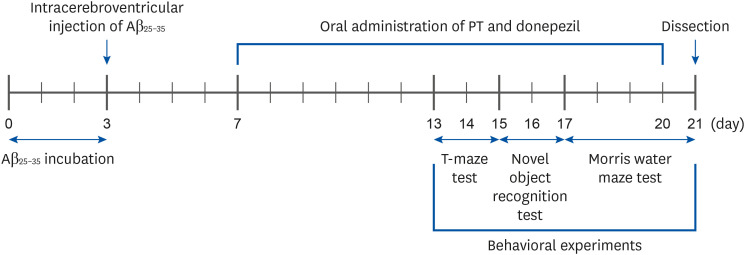
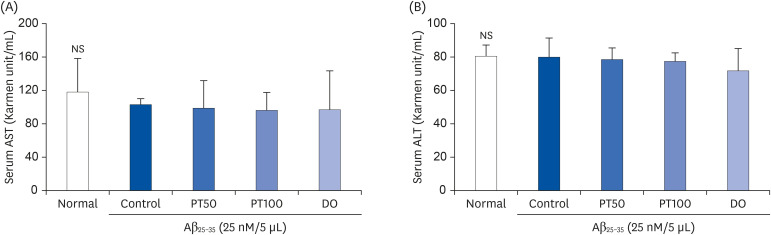
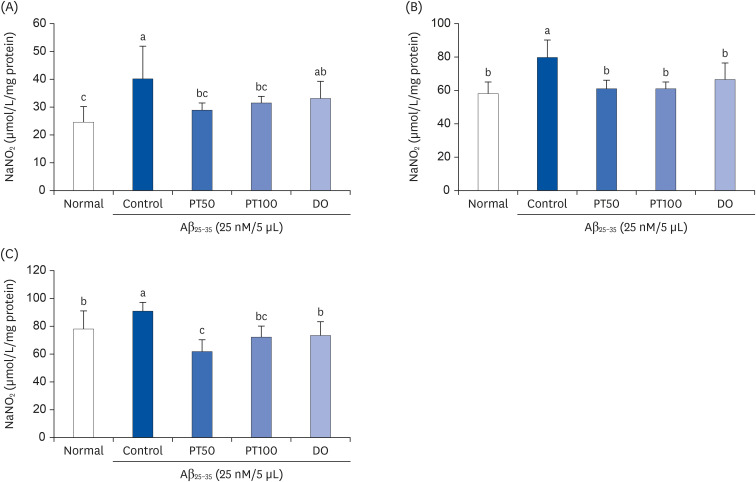
We orally administered PT (50 and 100 mg/kg/day) for 14 days in an Aβ 25–35-induced mouse model and conducted behavioral experiments to test cognitive ability. In addition, we evaluated the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum and measured the production of lipid peroxide, nitric oxide (NO), and reactive oxygen species (ROS) in tissues.
RESULTS
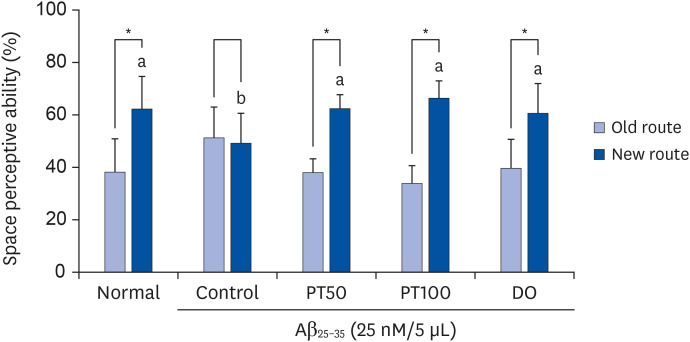
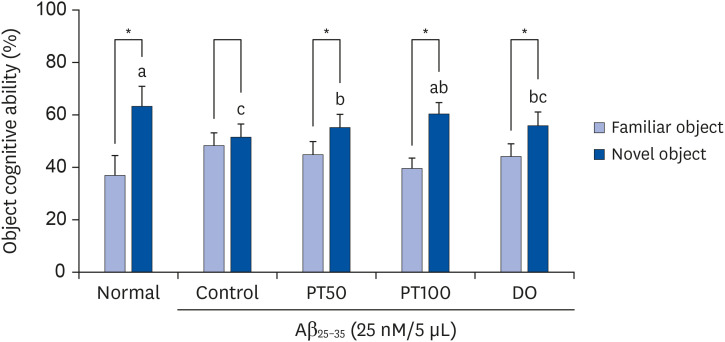
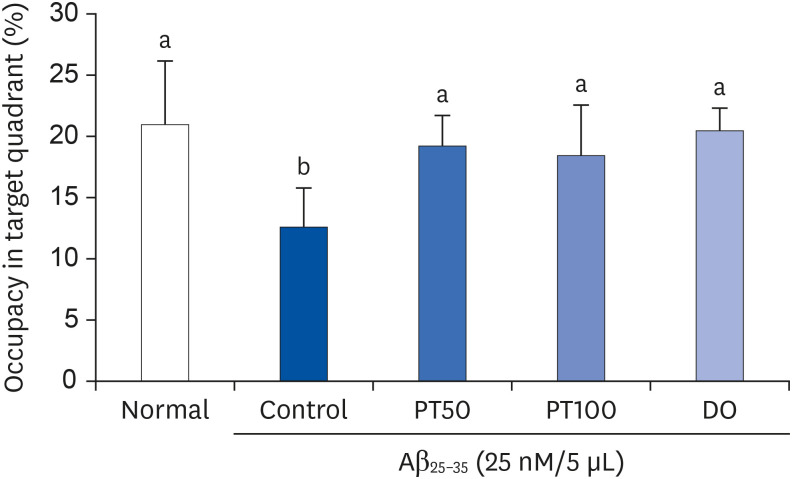
PT treatment improved the space perceptive ability in the T-maze test, object cognitive ability in the novel object recognition test, and spatial learning/long-term memory in the Morris water-maze test. Moreover, the levels of AST and ALT were not significantly different among the groups, indicating that PT did not show liver toxicity. Furthermore, administration of PT significantly inhibited the production of lipid peroxide, NO, and ROS in the brain, liver, and kidney, suggesting that PT protected against oxidative stress.
CONCLUSIONS
Our study demonstrated that administration of PT improved Aβ25–35 -induced cognitive impairment by regulating oxidative stress. Therefore, we propose that PT could be used as a natural agent for AD improvement.
Figure
Reference
-
1. Jahn H. Memory loss in Alzheimer's disease. Dialogues Clin Neurosci. 2013; 15:445–454. PMID: 24459411.2. Derby CA. Trends in the public health significance, definitions of disease, and implications for prevention of Alzheimer's disease. Curr Epidemiol Rep. 2020; 7:68–76.3. Zhao N, Ren Y, Yamazaki Y, Qiao W, Li F, Felton LM, Mahmoudiandehkordi S, Kueider-Paisley A, Sonoustoun B, Arnold M, et al. Alzheimer's Risk Factors Age, APOE Genotype, and Sex Drive Distinct Molecular Pathways. Neuron. 2020; 106:727–742.e6. PMID: 32199103.4. Oudin A. Short review: air pollution, noise and lack of greenness as risk factors for Alzheimer's disease- epidemiologic and experimental evidence. Neurochem Int. 2020; 134:104646. PMID: 31866324.5. Götz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004; 9:664–683. PMID: 15052274.6. Jakob-Roetne R, Jacobsen H. Alzheimer's disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl. 2009; 48:3030–3059. PMID: 19330877.7. Dhitavat S, Rivera ER, Rogers E, Shea TB. Differential efficacy of lipophilic and cytosolic antioxidants on generation of reactive oxygen species by amyloid-β. J Alzheimers Dis. 2001; 3:525–529.8. De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007; 282:11590–11601. PMID: 17308309.9. Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006; 3:327–337. PMID: 17017863.10. Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004; 72:111–127. PMID: 15063528.11. Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005; 105:145–158. PMID: 15863734.12. Kwak JH, Oh YS. Evaluation of Populus alba × glandulosa as raw material of particleboard. J Korean For Soc. 2003; 92:140–144.13. Choi SI, Hwang SJ, Lee OH, Kim JD. Antioxidant activity and component analysis of Populus tomentiglandulosa extract. Korean J Food Sci Technol. 2020; 52:119–124.14. Dudonné S, Poupard P, Coutière P, Woillez M, Richard T, Mérillon JM, Vitrac X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J Agric Food Chem. 2011; 59:4527–4536. PMID: 21425781.15. Lee HJ, Kim JS, Kim YK, Ryu JH. Phenolic glycosides as inhibitors of inducible nitric oxide synthase from Populus davidiana in LPS-activated RAW 264.7 murine macrophages. Pharmazie. 2012; 67:870–873. PMID: 23136723.16. Debbache-Benaida N, Atmani-Kilani D, Schini-Keirth VB, Djebbli N, Atmani D. Pharmacological potential of Populus nigra extract as antioxidant, anti-inflammatory, cardiovascular and hepatoprotective agent. Asian Pac J Trop Biomed. 2013; 3:697–704. PMID: 23998009.17. Lee CH, Park JH, Ahn JH, Kim JD, Cho JH, Lee TK, Won MH. Stronger antioxidant enzyme immunoreactivity of Populus tomentiglandulosa extract than ascorbic acid in rat liver and kidney. Iran J Basic Med Sci. 2019; 22:963–967. PMID: 31579454.18. Park JH, Lee TK, Ahn JH, Shin BN, Cho JH, Kim IH, Lee JC, Kim JD, Lee YJ, Kang IJ, et al. Pre-treated Populus tomentiglandulosa extract inhibits neuronal loss and alleviates gliosis in the gerbil hippocampal CA1 area induced by transient global cerebral ischemia. Anat Cell Biol. 2017; 50:284–292. PMID: 29354300.19. Glascock JJ, Osman EY, Coady TH, Rose FF, Shababi M, Lorson CL. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp. 2011; 56:2968.20. Kim JH, He MT, Kim MJ, Yang CY, Shin YS, Yokozawa T, Park CH, Cho EJ. Safflower (Carthamus tinctorius L.) seed attenuates memory impairment induced by scopolamine in mice via regulation of cholinergic dysfunction and oxidative stress. Food Funct. 2019; 10:3650–3659. PMID: 31165850.21. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006; 1:1306–1311. PMID: 17406415.22. He MT, Lee AY, Kim JH, Park CH, Shin YS, Cho EJ. Protective role of Cordyceps militaris in Aβ1-42-induced Alzheimer's disease in vivo . Food Sci Biotechnol. 2018; 28:865–872. PMID: 31093445.23. Jang WS, Choung SY. Antiobesity effects of the ethanol extract of Laminaria japonica Areshoung in high-fat-diet-induced obese rat. Evid Based Complement Alternat Med. 2013; 2013:492807. PMID: 23365609.24. Kim JH, Lee J, Lee S, Cho EJ. Quercetin and quercetin-3-β-D-glucoside improve cognitive and memory function in Alzheimer's disease mouse. Appl Biol Chem. 2016; 59:721–728.25. Lee AY, Choi JM, Lee J, Lee MH, Lee S, Cho EJ. Effects of vegetable oils with different fatty acid compositions on cognition and memory ability in Aβ25–35-induced Alzheimer's disease mouse model. J Med Food. 2016; 19:912–921. PMID: 27696934.26. Park CH, Lee AY, Kim JH, Seong SH, Cho EJ, Choi JS, Kim MJ, Yang S, Yokozawa T, Shin YS. Protective effects of serotonin and its derivatives, N-feruloylserotonin and N-(p-coumaroyl) serotonin, against cisplatin-induced renal damage in mice. Am J Chin Med. 2019; 47:369–383. PMID: 30827154.27. Chauhan V, Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology. 2006; 13:195–208. PMID: 16781128.28. Ovchinnikova OY, Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The Osaka FAD mutation E22Δ leads to the formation of a previously unknown type of amyloid β fibrils and modulates Aβ neurotoxicity. J Mol Biol. 2011; 408:780–791. PMID: 21402079.29. Canevari L, Abramov AY, Duchen MR. Toxicity of amyloid β peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res. 2004; 29:637–650. PMID: 15038611.30. Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 2010; 16:2766–2778. PMID: 20698820.31. Tarus B, Nguyen PH, Berthoumieu O, Faller P, Doig AJ, Derreumaux P. Molecular structure of the NQTrp inhibitor with the Alzheimer Aβ1-28 monomer. Eur J Med Chem. 2015; 91:43–50. PMID: 25011560.32. Lee YW, Kim DH, Jeon SJ, Park SJ, Kim JM, Jung JM, Lee HE, Bae SG, Oh HK, Son KH, et al. Neuroprotective effects of salvianolic acid B on an Aβ25-35 peptide-induced mouse model of Alzheimer's disease. Eur J Pharmacol. 2013; 704:70–77. PMID: 23461850.33. Liu RT, Zou LB, Lü QJ. Liquiritigenin inhibits Abeta(25-35)-induced neurotoxicity and secretion of Abeta(1-40) in rat hippocampal neurons. Acta Pharmacol Sin. 2009; 30:899–906. PMID: 19574995.34. Liu YM, Li ZY, Hu H, Xu SP, Chang Q, Liao YH, Pan RL, Liu XM. Tenuifolin, a secondary saponin from hydrolysates of polygalasaponins, counteracts the neurotoxicity induced by Aβ25-35 peptides in vitro and in vivo . Pharmacol Biochem Behav. 2015; 128:14–22. PMID: 25444865.35. Mabunga DF, Park D, Ryu O, Valencia ST, Adil KJ, Kim S, Kwon KJ, Shin CY, Jeon SJ. Recapitulation of neuropsychiatric behavioral features in mice using acute low-dose MK-801 administration. Exp Neurobiol. 2019; 28:697–708. PMID: 31902157.36. Hsieh LS, Wen JH, Miyares L, Lombroso PJ, Bordey A. Outbred CD1 mice are as suitable as inbred C57BL/6J mice in performing social tasks. Neurosci Lett. 2017; 637:142–147. PMID: 27871995.37. Lee AY, Choi JM, Lee YA, Shin SH, Cho EJ. Beneficial effect of black rice (Oryza sativa L. var. japonica) extract on amyloid β-induced cognitive dysfunction in a mouse model. Exp Ther Med. 2020; 20:64. PMID: 32963594.38. Cho CH, Jung YS, Kim JM, Nam TG, Lee SH, Cho HS, Song MC, Heo HJ, Kim DO. Neuroprotective effects of Actinidia eriantha cv. Bidan kiwifruit on amyloid beta-induced neuronal damages in PC-12 cells and ICR mice. J Funct Foods. 2021; 79:104398.39. Cacabelos R. Donepezil in Alzheimer's disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007; 3:303–333. PMID: 19300564.40. Dooley M, Lamb HM. Donepezil: a review of its use in Alzheimer's disease. Drugs Aging. 2000; 16:199–226. PMID: 10803860.41. Mehta M, Adem A, Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer's disease. Int J Alzheimers Dis. 2012; 2012:728983. PMID: 22216416.42. Umukoro S, Adewole FA, Eduviere AT, Aderibigbe AO, Onwuchekwa C. Free radical scavenging effect of donepezil as the possible contribution to its memory enhancing activity in mice. Drug Res (Stuttg). 2014; 64:236–239. PMID: 24203083.43. Kim HG, Moon M, Choi JG, Park G, Kim AJ, Hur J, Lee KT, Oh MS. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo . Neurotoxicology. 2014; 40:23–32. PMID: 24189446.44. Choi SI, Hwang SJ, Lee OH, Kim JD. Antioxidant activity and component analysis of Populus tomentiglandulosa extract. Korean J Food Sci Technol. 2020; 52:119–124.45. He J, Xu L, Yang L, Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med Sci Monit. 2018; 24:8198–8206. PMID: 30428482.46. Lim HJ, Shim SB, Jee SW, Lee SH, Lim CJ, Hong JT, Sheen YY, Hwang DY. Green tea catechin leads to global improvement among Alzheimer's disease-related phenotypes in NSE/hAPP-C105 Tg mice. J Nutr Biochem. 2013; 24:1302–1313. PMID: 23333093.47. Wenk GL. Assessment of spatial memory using the T maze. Curr Protoc Neurosci. 1998; 4:8.5B.1.48. McHugh SB, Niewoehner B, Rawlins JN, Bannerman DM. Dorsal hippocampal N-methyl-D-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the T-maze. Behav Brain Res. 2008; 186:41–47. PMID: 17868929.49. Choi YY, Maeda T, Fujii H, Yokozawa T, Kim HY, Cho EJ, Shibamoto T. Oligonol improves memory and cognition under an amyloid β(25-35)-induced Alzheimer's mouse model. Nutr Res. 2014; 34:595–603. PMID: 25150118.50. Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. J Alzheimers Dis. 2019; 68:1699–1710. PMID: 30958364.51. Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006; 1:7–12. PMID: 17406205.52. Lee AY, Hwang BR, Lee MH, Lee S, Cho EJ. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25-35 induced impairment of cognition and memory function. Nutr Res Pract. 2016; 10:274–281. PMID: 27247723.53. Denninger JK, Smith BM, Kirby ED. Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp. 2018; e58593.54. Bengoetxea X, Rodriguez-Perdigon M, Ramirez MJ. Object recognition test for studying cognitive impairments in animal models of Alzheimer's disease. Front Biosci (Schol Ed). 2015; 7:10–29. PMID: 25961683.55. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017; e55718.56. Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, Zou LB, Nabeshima T. Xanthoceraside attenuates amyloid β peptide25−35-induced learning and memory impairments in mice. Psychopharmacology (Berl). 2012; 219:181–190. PMID: 21735075.57. Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. 2011; 53:e2920.58. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1:848–858. PMID: 17406317.59. Tian H, Ding N, Guo M, Wang S, Wang Z, Liu H, Yang J, Li Y, Ren J, Jiang J, et al. Analysis of learning and memory ability in an Alzheimer's disease mouse model using the Morris water maze. J Vis Exp. 2019; e60055.60. Niño SA, Morales-Martínez A, Chi-Ahumada E, Carrizales L, Salgado-Delgado R, Pérez-Severiano F, Díaz-Cintra S, Jiménez-Capdeville ME, Zarazúa S. Arsenic exposure contributes to the bioenergetic damage in an Alzheimer's disease model. ACS Chem Neurosci. 2019; 10:323–336. PMID: 30141907.61. Kwon SH, Lee HK, Kim JA, Hong SI, Kim SY, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, et al. Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta(25-35)-induced learning and memory impairments in mice. Neurosci Lett. 2011; 487:123–127. PMID: 20974223.62. Huang XJ, Choi YK, Im HS, Yarimaga O, Yoon E, Kim HS. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors (Basel). 2006; 6:756–782.63. Lee TK, Park JH, Ahn JH, Kim H, Song M, Lee JC, Kim JD, Jeon YH, Choi JH, Lee CH, et al. Pretreatment of Populus tomentiglandulosa protects hippocampal CA1 pyramidal neurons from ischemia-reperfusion injury in gerbils via increasing SODs expressions and maintaining BDNF and IGF-I expressions. Chin J Nat Med. 2019; 17:424–434. PMID: 31262455.64. Park JH, Lee TK, Ahn JH, Shin BN, Cho JH, Kim IH, Lee JC, Kim JD, Lee YJ, Kang IJ, et al. Pre-treated Populus tomentiglandulosa extract inhibits neuronal loss and alleviates gliosis in the gerbil hippocampal CA1 area induced by transient global cerebral ischemia. Anat Cell Biol. 2017; 50:284–292. PMID: 29354300.65. Lee CH, Park JH, Ahn JH, Kim JD, Cho JH, Lee TK, Won MH. Stronger antioxidant enzyme immunoreactivity of Populus tomentiglandulosa extract than ascorbic acid in rat liver and kidney. Iran J Basic Med Sci. 2019; 22:963–967. PMID: 31579454.66. Rajmohan R, Reddy PH. Amyloid-Beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer's disease neurons. J Alzheimers Dis. 2017; 57:975–999. PMID: 27567878.67. Wang D, Noda Y, Zhou Y, Mouri A, Mizoguchi H, Nitta A, Chen W, Nabeshima T. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine ameliorates the cognitive dysfunction in beta amyloid25-35 i.c.v.-injected mice: involvement of dopaminergic systems. Neuropsychopharmacology. 2007; 32:1261–1271. PMID: 17133263.68. Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, et al. Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol. 2014; 76:837–844. PMID: 25205593.69. Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS, Zhu XY, Giunta B, Tan J, Song WH, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer's disease. Acta Neuropathol. 2015; 130:487–499. PMID: 26363791.70. Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002; 32:1050–1060. PMID: 12031889.71. Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ 2nd, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002; 33:620–626. PMID: 12208348.72. Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004; 57:453–455. PMID: 15765761.73. Choi SY, Lee J, Lee DG, Lee S, Cho EJ. Acer okamotoanum improves cognition and memory function in Aβ25–35-induced Alzheimer's mice model. Appl Biol Chem. 2017; 60:1–9.74. Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-β peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998; 785:195–206. PMID: 9518610.75. Law A, Gauthier S, Quirion R. Say NO to Alzheimer's disease: the putative links between nitric oxide and dementia of the Alzheimer's type. Brain Res Brain Res Rev. 2001; 35:73–96. PMID: 11245887.76. Wei T, Chen C, Hou J, Xin W, Mori A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta. 2000; 1498:72–79. PMID: 11042352.77. Dubey M, Nagarkoti S, Awasthi D, Singh AK, Chandra T, Kumaravelu J, Barthwal MK, Dikshit M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016; 7:e2348. PMID: 27584786.78. Díaz A, De Jesús L, Mendieta L, Calvillo M, Espinosa B, Zenteno E, Guevara J, Limón ID. The amyloid-beta25-35 injection into the CA1 region of the neonatal rat hippocampus impairs the long-term memory because of an increase of nitric oxide. Neurosci Lett. 2010; 468:151–155. PMID: 19879921.79. Ryu JH, Ahn H, Kim JY, Kim YK. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytother Res. 2003; 17:485–489. PMID: 12748984.80. Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997; 23:134–147. PMID: 9165306.81. Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018; 14:450–464. PMID: 29080524.82. Díaz A, Treviño S, Pulido-Fernandez G, Martínez-Muñoz E, Cervantes N, Espinosa B, Rojas K, Pérez-Severiano F, Montes S, Rubio-Osornio M, et al. Epicatechin reduces spatial memory deficit caused by amyloid-β25–35 toxicity modifying the heat shock proteins in the CA1 region in the hippocampus of rats. Antioxidants. 2019; 8:113.83. Leutner S, Eckert A, Müller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm (Vienna). 2001; 108:955–967. PMID: 11716148.84. Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U. oxidant/antioxidant imbalance in Alzheimer's disease: therapeutic and diagnostic prospects. Oxid Med Cell Longev. 2018; 2018:6435861. PMID: 29636850.85. Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007; 8:101–112. PMID: 17245412.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the fermented Zizyphus jujuba in the amyloid β25-35 -induced Alzheimer's disease mouse model
- Protective role of caffeic acid in an Abeta25-35-induced Alzheimer's disease model
- Protective Effect of Fibroin BF-7 on Neuronal Cell Death in Alzheimer Model using Amyloid beta Peptide
- Protective Effect of Rice Bran Oil against β-Amyloid Protein-Induced Memory Impairment and Neuronal Death in Mice
- The association between PGC-1a and Alzheimer's disease