Nutr Res Pract.
2021 Apr;15(2):173-186. 10.4162/nrp.2021.15.2.173.
Effects of the fermented Zizyphus jujuba in the amyloid β25-35 -induced Alzheimer's disease mouse model
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busan 46241, Korea
- 2Department of Plant Science and Technology, Chung-Ang University, Anseong 17546, Korea
- 3Department of Food Science, Gyeongnam National University of Science and Technology, Jinju 52725, Korea
- KMID: 2514442
- DOI: http://doi.org/10.4162/nrp.2021.15.2.173
Abstract
- BACKGROUD/OBJECTIVES: Alzheimer's disease (AD) is the most common cause of dementia in the elderly. Due to the increased incidence of dementia, there is a corresponding increase concerning the importance of AD. In this study, we investigated the protective effects conferred by Zizyphus jujuba (Zj) and Zizyphus jujuba fermented by yeast (Zj-Y), on cognitive impairment in an AD mouse model.
MATERIALS/METHODS
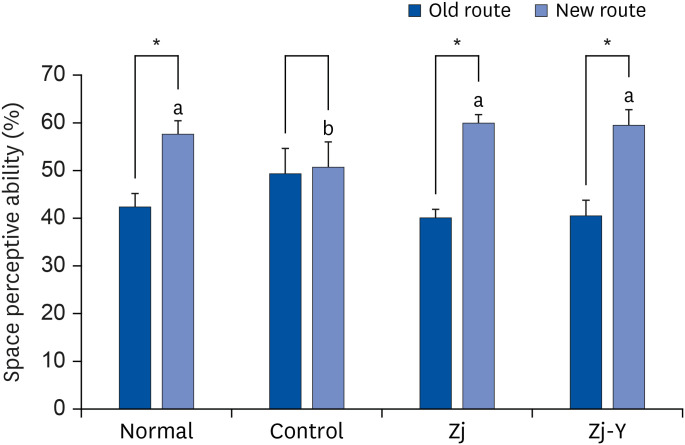
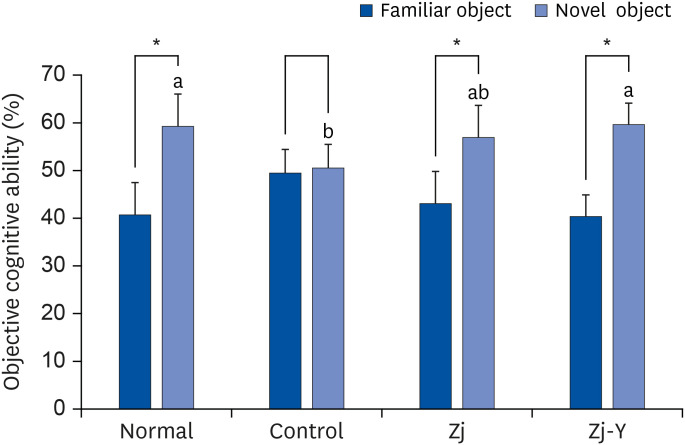
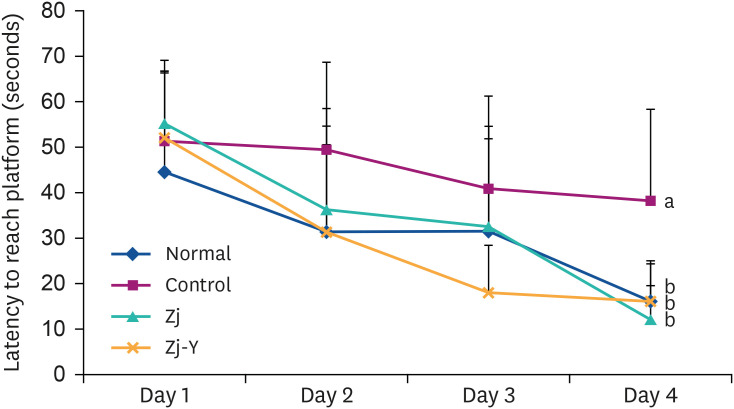
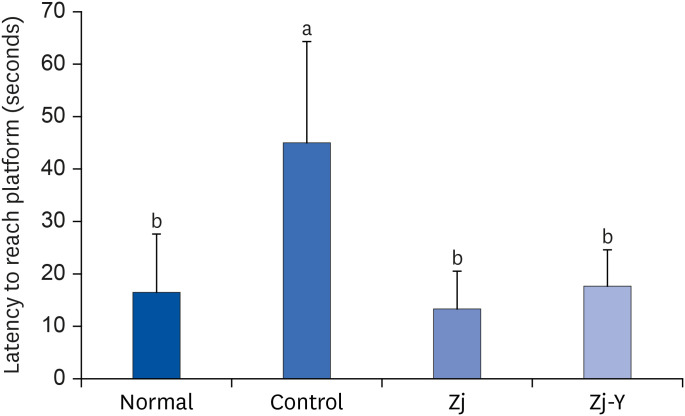
AD was induced by injecting amyloid beta25-35 (Aβ25-35 ) in ICR mice, and subsequently 200 mg/kg Zj or Zj-Y was administered daily for 14 days. The cognitive ability of AD mice was observed through behavioral experiments in T-maze, novel object recognition, and Morris water maze tests. We subsequently measured the levels of malondialdehyde (MDA), nitric oxide (NO), aspartate aminotransferase, and alanine aminotransferase in either tissues or serum.
RESULTS
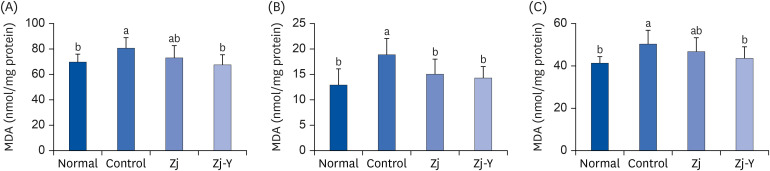
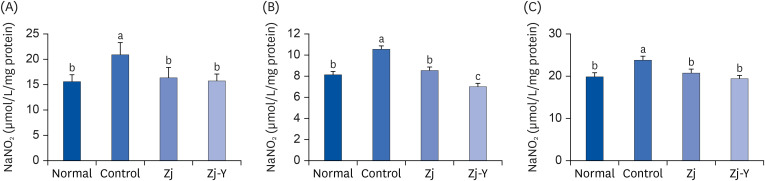
In behavioral tests, deterioration was revealed in the short- and long-term learning and memory functions in the Aβ25-35 -injected control group compared to the normal group, indicating that Aβ25-35 injection impairs cognitive functions. However, administration of Zj and Zj-Y improved cognitive function in mice, as compared to the Aβ25-35 -injected control mice. In addition, the Aβ25-35 induced elevations of MDA and NO in the brain, kidney, and liver were suppressed after exposure to Zj and Zj-Y. Especially, Zj-Y showed stronger scavenging effect against MDA and NO, as compared to Zj.
CONCLUSIONS
Results of the present study indicate that Zj-Y exerts a protective effect on cognitive impairment and memory dysfunction, which is exerted by attenuating the oxidative stress induced by Aβ25-35 .
Keyword
Figure
Reference
-
1. Tsunekawa H, Noda Y, Mouri A, Yoneda F, Nabeshima T. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25-35). Behav Brain Res. 2008; 190:224–232. PMID: 18420288.
Article2. Moreira PI. Alzheimer's disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. J Alzheimers Dis. 2012; 30(Suppl 2):S199–S215. PMID: 22269163.
Article3. Coley N, Andrieu S, Gardette V, Gillette-Guyonnet S, Sanz C, Vellas B, Grand A. Dementia prevention: methodological explanations for inconsistent results. Epidemiol Rev. 2008; 30:35–66. PMID: 18779228.
Article4. Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001; 81:741–766. PMID: 11274343.
Article5. Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008; 14:45–53. PMID: 18218341.
Article6. Chang SC, Hsu BY, Chen BH. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int J Biol Macromol. 2010; 47:445–453. PMID: 20615429.7. Li J, Liu Y, Fan L, Ai L, Shan L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr Polym. 2011; 84:390–394.8. Jung JE, Cho EJ. Protective effects of Zizyphus jujuba and fermented Zizyphus jujuba from free radicals and hair loss. J Korean Soc Food Sci Nutr. 2014; 43:1174–1180.9. Yu L, Jiang BP, Luo D, Shen XC, Guo S, Duan JA, Tang YP. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine. 2012; 19:239–244. PMID: 21982434.10. Kaeidi A, Taati M, Hajializadeh Z, Jahandari F, Rashidipour M. Aqueous extract of Zizyphus jujuba fruit attenuates glucose induced neurotoxicity in an in vitro model of diabetic neuropathy. Iran J Basic Med Sci. 2015; 18:301–306. PMID: 25945244.11. Gao QH, Wu CS, Wang M. The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J Agric Food Chem. 2013; 61:3351–3363. PMID: 23480594.12. Zhang Y, Yang X, Jin G, Yang X, Zhang Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer's disease. Int J Biol Macromol. 2016; 92:935–941. PMID: 27498414.13. Deng LL, Yuan D, Zhou ZY, Wan JZ, Zhang CC, Liu CQ, Dun YY, Zhao HX, Zhao B, Yang YJ, Wang T. Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogen-activated protein kinase and nuclear factor kappa B signaling pathways. Neural Regen Res. 2017; 12:1877–1884. PMID: 29239335.14. Stanton C, Ross RP, Fitzgerald GF, Van Sinderen D. Fermented functional foods based on probiotics and their biogenic metabolites. Curr Opin Biotechnol. 2005; 16:198–203. PMID: 15831387.
Article15. Gumienna M, Szwengiel A, Górna B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur Food Res Technol. 2016; 242:631–640.
Article16. Milani EA, Silva FV. Ultrasound assisted thermal pasteurization of beers with different alcohol levels: Inactivation of Saccharomyces cerevisiae ascospores. J Food Eng. 2017; 198:45–53.17. Struyf N, Laurent J, Verspreet J, Verstrepen KJ, Courtin CM. Substrate-limited Saccharomyces cerevisiae yeast strains allow control of fermentation during bread making. J Agric Food Chem. 2017; 65:3368–3377. PMID: 28367622.18. Ju HK, Cho EJ, Jang MH, Lee YY, Hong SS, Park JH, Kwon SW. Characterization of increased phenolic compounds from fermented Bokbunja (Rubus coreanus Miq.) and related antioxidant activity. J Pharm Biomed Anal. 2009; 49:820–827. PMID: 19179032.19. Jung YM, Lee SH, Lee DS, You MJ, Chung IK, Cheon WH, Kwon YS, Lee YJ, Ku SK. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011; 31:387–396. PMID: 21636017.
Article20. Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009; 112:858–862.21. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357. PMID: 3784576.22. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293. PMID: 14946277.
Article23. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006; 1:1306–1311. PMID: 17406415.
Article24. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60. PMID: 6471907.
Article25. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86:271–278. PMID: 655387.26. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.27. Schmidt HH, Warner TD, Nakane M, Förstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992; 41:615–624. PMID: 1373797.28. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28:56–63. PMID: 13458125.
Article29. Woo M, Noh JS, Cho EJ, Song YO. Bioactive compounds of kimchi inhibit apoptosis by attenuating endoplasmic reticulum stress in the brain of amyloid β-injected mice. J Agric Food Chem. 2018; 66:4883–4890. PMID: 29706080.
Article30. Shen X, Tang Y, Yang R, Yu L, Fang T, Duan JA. The protective effect of Zizyphus jujube fruit on carbon tetrachloride-induced hepatic injury in mice by anti-oxidative activities. J Ethnopharmacol. 2009; 122:555–560. PMID: 19429327.31. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006; 368:387–403. PMID: 16876668.
Article32. Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, Zou LB, Nabeshima T. Xanthoceraside attenuates amyloid β peptide25−35-induced learning and memory impairments in mice. Psychopharmacology (Berl). 2012; 219:181–190. PMID: 21735075.33. Lee AY, Hwang BR, Lee MH, Lee S, Cho EJ. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25-35 induced impairment of cognition and memory function. Nutr Res Pract. 2016; 10:274–281. PMID: 27247723.34. Kim JH, Lee JM, Lee SH, Cho EJ. Quercetin and quercetin-3-β-d-glucoside improve cognitive and memory function in Alzheimer's disease mouse. Appl Biol Chem. 2016; 59:721–728.
Article35. Splittstoesser DF. Microorganisms involved in the spoilage of fermented fruit juices. J Food Prot. 1982; 45:874–877. PMID: 30866300.
Article36. Romero AM, Doval MM, Sturla MA, Judis MA. Antioxidant properties of polyphenol-containing extract from soybean fermented with Saccharomyces cerevisiae . Eur J Lipid Sci Technol. 2004; 106:424–431.37. Zhao L, Liu F, Wu L, Xue X, Hou F. Fate of triadimefon and its metabolite triadimenol in jujube samples during jujube wine and vinegar processing. Food Control. 2017; 73:468–473.38. Jung SW, Noh WS. The effect of jujube extract on the growth of lactic acid bacteria. J East Asian Soc Diet Life. 2006; 16:349–356.39. Eom IJ, Choi JI, Kim IH, Kim TH, Kim SH. Changes in the physicochemical and antioxidant characteristics during the fermentation of jujube wine using hot water extract of dried jujube . J Life Sci. 2016; 26:1298–1307.40. Jung JE. The protective effects of Zizyphus jujuba and fermented Zizyphus jujuba from oxidative stress, recognition impairment and hair loss [Master's thesis]. Busan: Pusan National University;2011.41. Ghaly IS, Said A, Abdel-Wahhab MA. Zizyphus jujuba and Origanum majorana extracts protect against hydroquinone-induced clastogenicity. Environ Toxicol Pharmacol. 2008; 25:10–19. PMID: 21783830.42. Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004; 28:699–709. PMID: 15555679.
Article43. Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995; 16:149–160. PMID: 7777133.
Article44. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012; 13:93–110. PMID: 22160349.
Article45. Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002; 5:868–873. PMID: 12195431.
Article46. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1:848–858. PMID: 17406317.
Article47. Rabiei Z, Rafieian-Kopaei M, Heidarian E, Saghaei E, Mokhtari S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem Res. 2014; 39:353–360. PMID: 24379110.48. Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002; 32:1050–1060. PMID: 12031889.49. Tullberg C, Larsson K, Carlsson NG, Comi I, Scheers N, Vegarud G, Undeland I. Formation of reactive aldehydes (MDA, HHE, HNE) during the digestion of cod liver oil: comparison of human and porcine in vitro digestion models. Food Funct. 2016; 7:1401–1412. PMID: 26838473.50. Fonseca I, Reguengo H, Almeida M, Dias L, Martins LS, Pedroso S, Santos J, Lobato L, Henriques AC, Mendonça D. Oxidative stress in kidney transplantation: malondialdehyde is an early predictive marker of graft dysfunction. Transplantation. 2014; 97:1058–1065. PMID: 24406454.51. Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D, Oettl K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radic Res. 2008; 42:633–638. PMID: 18654878.
Article52. Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to β-amyloid plaques. Exp Neurol. 1997; 144:266–272. PMID: 9168828.
Article53. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–37. PMID: 8944624.
Article54. Law A, Gauthier S, Quirion R. Neuroprotective and neurorescuing effects of isoform-specific nitric oxide synthase inhibitors, nitric oxide scavenger, and antioxidant against beta-amyloid toxicity. Br J Pharmacol. 2001; 133:1114–1124. PMID: 11487523.
Article55. Jung JE, Cho EJ. Enhancement of anti-inflammatory effect of Zizyphus jujuba var. inermis fruits by fermentation. Cancer Prev Res. 2011; 16:263–268.56. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999; 45:129–133. PMID: 10369716.
Article57. Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008; 47:1363–1370. PMID: 18366115.
Article58. Chen J, Li Z, Maiwulanjiang M, Zhang WL, Zhan JY, Lam CT, Zhu KY, Yao P, Choi RC, Lau DT, Dong TT, Tsim KW. Chemical and biological assessment of Ziziphus jujuba fruits from China: different geographical sources and developmental stages. J Agric Food Chem. 2013; 61:7315–7324. PMID: 23841724.59. Kwon H, Jung IH, Yi JH, Kim JH, Park JH, Lee S, Jung JW, Lee YC, Ryu JH, Kim DH. The seed of Zizyphus jujuba var. spinosa attenuates Alzheimer's disease-associated hippocampal synaptic deficits through BDNF/TrkB signaling. Biol Pharm Bull. 2017; 40:2096–2104. PMID: 29199234.60. Ko SY, Lee HE, Park SJ, Jeon SJ, Kim B, Gao Q, Jang DS, Ryu JH. Spinosin, a C-Glucosylflavone, from Zizyphus jujuba var. spinosa ameliorates Aβ1–42 oligomer-induced memory impairment in mice. Biomol Ther. 2015; 23:156–164.61. Chen J, Maiwulanjiang M, Lam KY, Zhang WL, Zhan JY, Lam CT, Xu SL, Zhu KY, Yao P, Lau DT, Dong TT, Tsim KW. A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: a signaling mediated by protein kinase A. J Agric Food Chem. 2014; 62:1890–1897. PMID: 24520858.62. Chen J, Yan AL, Lam KY, Lam CT, Li N, Yao P, Xiong A, Dong TT, Tsim KW. A chemically standardized extract of Ziziphus jujuba fruit (Jujube) stimulates expressions of neurotrophic factors and anti-oxidant enzymes in cultured astrocytes. Phytother Res. 2014; 28:1727–1730. PMID: 25066116.63. Hwang IK, Yoo KY, Yoo DY, Choi JH, Lee CH, Kang IJ, Kwon DY, Kim YS, Kim DW, Won MH. Zizyphus enhances cell proliferation and neuroblast differentiation in the subgranular zone of the dentate gyrus in middle-aged mice. J Med Food. 2011; 14:195–200. PMID: 21332397.64. Chen J, Liu X, Li Z, Qi A, Yao P, Zhou Z, Dong TT, Tsim KW. A review of dietary Ziziphus jujuba Fruit (Jujube): developing health food supplements for brain protection. Evid Based Complement Alternat Med. 2017; 2017:3019568. PMID: 28680447.65. Kim NM, Lee JW, Do JH, Park CK, Yang JW. Effects of the fermentation periods on the qualities and functionality of the vegetable fermented broth. Korean J Med Crop Sci. 2005; 13:293–299.66. Hyon JS, Kang SM, Han SW, Kang MC, Oh MC, Oh CK, Kim DW, Jeon YJ, Kim SH. Flavonoid component changes and antioxidant activities of fermented Citrus gradis osbeck peel. J Korean Soc Food Sci Nutr. 2009; 38:1310–1316.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinosin, a C-Glucosylflavone, from Zizyphus jujuba var. spinosa Ameliorates Abeta1-42 Oligomer-Induced Memory Impairment in Mice
- In vivo Image of Cerebral Amyloid Angiopathy in an Alzheimer's Disease Mouse Model
- Animal Models of Alzheimer's Dementia
- Protective Effect of Rice Bran Oil against β-Amyloid Protein-Induced Memory Impairment and Neuronal Death in Mice
- Protective Effect of Ginsenoside Rb1 and Rg1 Against beta Amyloid ( 25-35 )-Induced Neurotoxicity on B103 cells