Diabetes Metab J.
2022 Mar;46(2):307-318. 10.4093/dmj.2020.0287.
Iron Overload and the Risk of Diabetes in the General Population: Results of the Chinese Health and Nutrition Survey Cohort Study
- Affiliations
-
- 1Department of Nutrition and Food Hygiene, Zhejiang University School of Public Health, and Center of Clinical Big Data and Analytics of The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- KMID: 2527723
- DOI: http://doi.org/10.4093/dmj.2020.0287
Abstract
- Background
Recent studies have found that there are significant associations between body iron status and the development of diabetes. In the present study, we aimed to analyze the association among iron overload (IO), insulin resistance (IR), and diabetes in Chinese adults, and to explore the sex difference.
Methods
Men and women (age >19 years) who participated in the Chinese Health and Nutrition Survey and did not have diabetes at baseline were followed between 2009 and 2015 (n=5,779). Over a mean of 6 years, 75 participants were diagnosed with incident diabetes. Logistic regression was used to assess the risk factors associated with IO. Cox proportional hazard regression was used to estimate the risk of incident diabetes and to determine whether the risk differed among subgroups. Causal mediation analysis (CMA) was used to explore the mechanism linking IO and diabetes.
Results
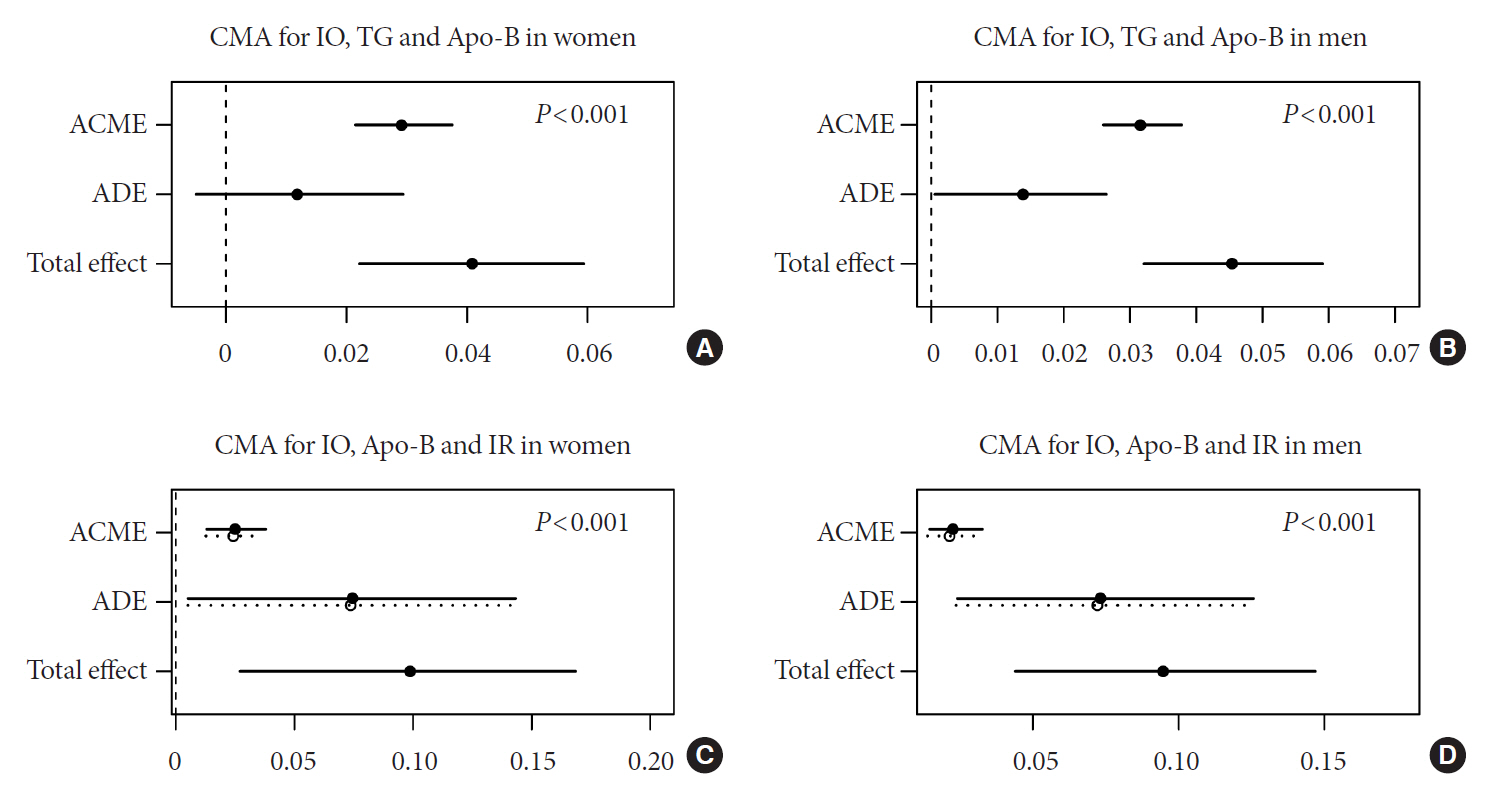
According to sex-stratified multivariable-adjusted Cox proportional hazards regression, IO increased the risk of incident diabetes. Women with IO had a higher risk of diabetes than men. Subgroup analysis with respect to age showed that the association between IO and diabetes was stronger in older women and younger men (P<0.001). CMA showed that liver injury (alanine transaminase) and lipid metabolism abnormalities (triglyceride, apolipoprotein B) contributed to the association between IO and diabetes.
Conclusion
IO is associated with diabetes and this association is sex-specific. IO may indirectly induce IR via liver injury and lipid metabolism abnormalities, resulting in diabetes.
Keyword
Figure
Reference
-
1. Eshak ES, Iso H, Maruyama K, Muraki I, Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin Nutr. 2018; 37:667–74.
Article2. Ikuta K, Hatayama M, Addo L, Toki Y, Sasaki K, Tatsumi Y, et al. Iron overload patients with unknown etiology from national survey in Japan. Int J Hematol. 2017; 105:353–60.
Article3. Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int. 2009; 3:434–44.
Article4. McElduff A. Iron: how much is too much? Diabetologia. 2017; 60:237–9.
Article5. Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. 2013; 17:329–41.
Article6. Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007; 30:1926–33.
Article7. Montonen J, Boeing H, Steffen A, Lehmann R, Fritsche A, Joost HG, et al. Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia. 2012; 55:2613–21.
Article8. Backe MB, Moen IW, Ellervik C, Hansen JB, MandrupPoulsen T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu Rev Nutr. 2016; 36:241–73.
Article9. Valenzuela R, Rincon-Cervera MA, Echeverria F, Barrera C, Espinosa A, Hernandez-Rodas MC, et al. Iron-induced prooxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition. 2018; 45:49–58.
Article10. Silva M, Silva ME, de Paula H, Carneiro CM, Pedrosa ML. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr Res. 2008; 28:391–8.
Article11. Jiang L, Wang K, Lo K, Zhong Y, Yang A, Fang X, et al. Sex-specific association of circulating ferritin level and risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab. 2019; 104:4539–51.
Article12. Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011; 60:1416–24.
Article13. Huth C, Beuerle S, Zierer A, Heier M, Herder C, Kaiser T, et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol. 2015; 173:643–53.
Article14. Han LL, Wang YX, Li J, Zhang XL, Bian C, Wang H, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014; 58:2189–95.
Article15. Podmore C, Meidtner K, Schulze MB, Scott RA, Ramond A, Butterworth AS, et al. Association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC-InterAct study. Diabetes Care. 2016; 39:572–81.
Article16. Zhang B, Zhai FY, Du SF, Popkin BM. The China Health and Nutrition Survey, 1989-2011. Obes Rev. 2014; 15 Suppl 1:2–7.
Article17. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV Infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization;2013. Chapter, Definition of key terms; p13-6 [cited 2021 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK374295.18. He J, Fang A, Yu S, Shen X, Li K. Dietary nonheme, heme, and total iron intake and the risk of diabetes in adults: results from the China Health and Nutrition Survey. Diabetes Care. 2020; 43:776–84.
Article19. Yu L, Yan J, Zhang Q, Lin H, Zhu L, Liu Q, et al. Association between serum ferritin and blood lipids: influence of diabetes and hs-CRP levels. J Diabetes Res. 2020; 2020:4138696.
Article20. Xu X, Byles JE, Shi Z, Hall JJ. Evaluation of older Chinese people’s macronutrient intake status: results from the China Health and Nutrition Survey. Br J Nutr. 2015; 113:159–71.
Article21. Batis C, Sotres-Alvarez D, Gordon-Larsen P, Mendez MA, Adair L, Popkin B. Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br J Nutr. 2014; 111:1441–51.
Article22. Zhan Y, Chen R, Zheng W, Guo C, Lu L, Ji X, et al. Association between serum magnesium and anemia: China health and nutrition survey. Biol Trace Elem Res. 2014; 159:39–45.
Article23. Li X, He T, Yu K, Lu Q, Alkasir R, Guo G, et al. Markers of iron status are associated with risk of hyperuricemia among Chinese adults: nationwide population-based study. Nutrients. 2018; 10:191.
Article24. WHO/UNICEF/UNU. Iron deficiency anaemia assessment, prevention and control. A guide for programme managers. Geneva: World Health Organization;2001. p. 33.25. StatPearls. Treasure Island. StatPearls Publishing;2020. Chapter, Iron overload [cited 2021 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526131.26. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003; 26:3320–5.
Article27. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001; 24:460–4.
Article28. Takahashi TA, Johnson KM. Menopause. Med Clin North Am. 2015; 99:521–34.
Article29. Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017; 318:2457–65.
Article30. Khan NA, Wang H, Anand S, Jin Y, Campbell NR, Pilote L, et al. Ethnicity and sex affect diabetes incidence and outcomes. Diabetes Care. 2011; 34:96–101.
Article31. Pitchika A, Schipf S, Nauck M, Dorr M, Lerch MM, Felix SB, et al. Associations of iron markers with type 2 diabetes mellitus and metabolic syndrome: results from the prospective SHIP study. Diabetes Res Clin Pract. 2020; 163:108149.
Article32. Jahng JW, Alsaadi RM, Palanivel R, Song E, Hipolito VE, Sung HK, et al. Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep. 2019; 20:e47911.
Article33. Weickert MO, Pfeiffer AF. Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetologia. 2006; 49:1732–41.
Article34. Hevi S, Chuck SL. Ferritins can regulate the secretion of apolipoprotein B. J Biol Chem. 2003; 278:31924–9.
Article35. Su Q, Tsai J, Xu E, Qiu W, Bereczki E, Santha M, et al. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009; 50:77–84.
Article36. Seki T, Kunichika T, Watanabe K, Orino K. Apolipoprotein B binds ferritin by hemin-mediated binding: evidence of direct binding of apolipoprotein B and ferritin to hemin. Biometals. 2008; 21:61–9.
Article37. Das SK, Patel VB, Basu R, Wang W, DesAulniers J, Kassiri Z, et al. Females are protected from iron-overload cardiomyopathy independent of iron metabolism: key role of oxidative stress. J Am Heart Assoc. 2017; 6:e003456.
Article38. Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009; 32:704–16.
Article39. Yang JH, Chou CH, Yang WS, Ho HN, Yang YS, Chen MJ. Iron stores and obesity are negatively associated with ovarian volume and anti-Mullerian hormone levels in women with polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2015; 54:686–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Iron and Its Significance in Obesity and Complications
- Molecular perspective of iron uptake, related diseases, and treatments
- Six Cases of Iron Containing Plasma Cells
- Diagnosis and treatment of transfusion-related iron overload
- Suicide Risk in Patients With Diabetes Varies by the Duration of Diabetes: The Korea National Health and Nutrition Examination Survey (2019)