Blood Res.
2019 Mar;54(1):10-16. 10.5045/br.2019.54.1.10.
Molecular perspective of iron uptake, related diseases, and treatments
- Affiliations
-
- 1Diagnostic Laboratory Sciences and Technology Research Center, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran. golafshansums@yahoo.com
- KMID: 2451050
- DOI: http://doi.org/10.5045/br.2019.54.1.10
Abstract
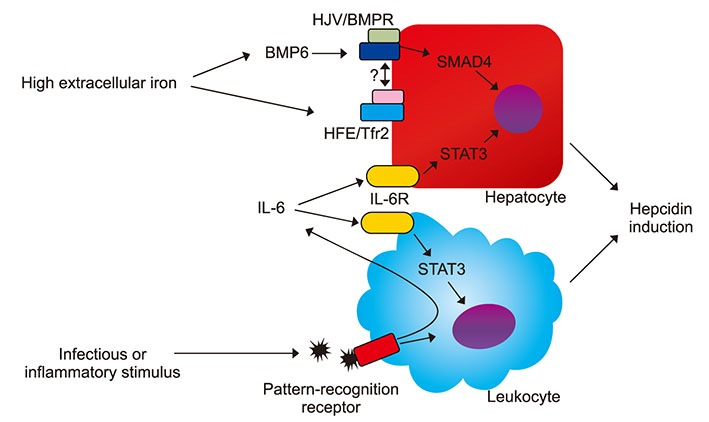
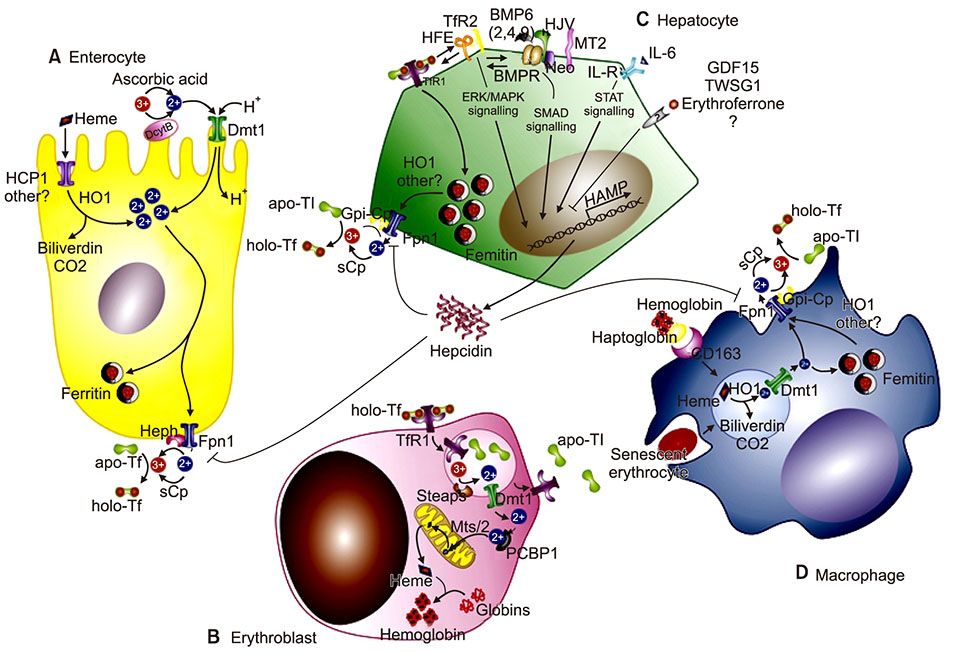
- Iron deficiency anemia and anemia of chronic disorders are the most common types of anemia. Disorders of iron metabolism lead to different clinical scenarios such as iron deficiency anemia, iron overload, iron overload with cataract and neurocognitive disorders. Regulation of iron in the body is a complex process and different regulatory proteins are involved in iron absorption and release from macrophages into hematopoietic tissues. Mutation in these regulatory genes is the most important cause of iron refractory iron deficiency anemia (IRIDA). This review provides a glance into the iron regulation process, diseases related to iron metabolism, and appropriate treatments at the molecular level.
MeSH Terms
Figure
Reference
-
1. Gurzau ES, Neagu C, Gurzau AE. Essential metals-case study on iron. Ecotoxicol Environ Saf. 2003; 56:190–200.
Article2. Goddard AF, James MW, McIntyre AS, Scott BB. British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011; 60:1309–1316.
Article3. Pandey R, Daloul R, Coyne DW. Iron treatment strategies in dialysis-dependent CKD. Semin Nephrol. 2016; 36:105–111.
Article4. Joosten E. Iron deficiency anemia in older adults: a review. Geriatr Gerontol Int. 2018; 18:373–379.
Article5. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014; 46:678–684.
Article6. Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015; 22:199–205.
Article7. Jiang X, Gao M, Chen Y, et al. EPO-dependent induction of erythroferrone drives hepcidin suppression and systematic iron absorption under phenylhydrazine-induced hemolytic anemia. Blood Cells Mol Dis. 2016; 58:45–51.
Article8. Kautz L, Jung G, Du X, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015; 126:2031–2037.
Article9. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015; 15:500–510.
Article10. Michels K, Nemeth E, Ganz T, Mehrad B. Hepcidin and host defense against infectious diseases. PLoS Pathog. 2015; 11:e1004998.
Article11. Arezes J, Nemeth E. Hepcidin and iron disorders: new biology and clinical approaches. Int J Lab Hematol. 2015; 37:92–98.
Article12. Wallace DF, McDonald CJ, Ostini L, Iser D, Tuckfield A, Subramaniam VN. The dynamics of hepcidin-ferroportin internalization and consequences of a novel ferroportin disease mutation. Am J Hematol. 2017; 92:1052–1061.
Article13. Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014; 28:671–681.
Article14. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004; 113:1271–1276.
Article15. Ganz T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005; 18:171–182.
Article16. Zoller H, Theurl I, Koch R, Kaser A, Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol Dis. 2002; 29:488–497.
Article17. Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2012; 1820:188–202.
Article18. Bali PK, Zak O, Aisen P. A new role for the transferrin receptor in the release of iron from transferrin. Biochemistry. 1991; 30:324–328.
Article19. Hamdi A, Roshan TM, Kahawita TM, Mason AB, Sheftel AD, Ponka P. Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run” mechanism. Biochim Biophys Acta. 2016; 1863:2859–2867.
Article20. Andriopoulos B Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009; 41:482–487.
Article21. Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006; 38:531–539.
Article22. Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl). 2009; 87:471–480.
Article23. Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009; 9:217–227.
Article24. Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008; 8:502–511.
Article25. Poli M, Luscieti S, Gandini V, et al. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica. 2010; 95:1832–1840.
Article26. Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematol Am Soc Hematol Educ Program. 2013; 2013:1–8.
Article27. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012; 1823:1434–1443.
Article28. Makis A, Hatzimichael E, Papassotiriou I, Voskaridou E. 2017 Clinical trials update in new treatments of β-thalassemia. Am J Hematol. 2016; 91:1135–1145.
Article29. Davis M, Clarke S. Influence of microRNA on the maintenance of human iron metabolism. Nutrients. 2013; 5:2611–2628.
Article30. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010; 11:597–610.
Article31. Zumbrennen-Bullough KB, Wu Q, Core AB, et al. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J Biol Chem. 2014; 289:23796–23808.
Article32. Joshi HP, Subramanian IV, Schnettler EK, et al. Dynamin 2 along with microRNA-199a reciprocally regulate hypoxia-inducible factors and ovarian cancer metastasis. Proc Natl Acad Sci U S A. 2014; 111:5331–5336.
Article33. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995; 270:1230–1237.
Article34. Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999; 341:1986–1995.
Article35. Bowes O, Baxter K, Elsey T, Snead M, Cox T. Hereditary hyperferritinaemia cataract syndrome. Lancet. 2014; 383:1520.
Article36. De Falco L, Sanchez M, Silvestri L, et al. Iron refractory iron deficiency anemia. Haematologica. 2013; 98:845–853.
Article37. Moirand R, Adams PC, Bicheler V, Brissot P, Deugnier Y. Clinical features of genetic hemochromatosis in women compared with men. Ann Intern Med. 1997; 127:105–110.
Article38. Adams PC, Deugnier Y, Moirand R, Brissot P. The relationship between iron overload, clinical symptoms, and age in 410 patients with genetic hemochromatosis. Hepatology. 1997; 25:162–166.
Article39. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011; 54:328–343.
Article40. Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003; 33:21–22.
Article41. Camaschella C, Roetto A, De Gobbi M. Juvenile hemochromatosis. Semin Hematol. 2002; 39:242–248.
Article42. Adams PC, Chakrabarti S. Genotypic/phenotypic correlations in genetic hemochromatosis: evolution of diagnostic criteria. Gastroenterology. 1998; 114:319–323.
Article43. Girelli D, Corrocher R, Bisceglia L, et al. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”). Blood. 1995; 86:4050–4053.
Article44. Panagiotou JP, Douros K. Clinicolaboratory findings and treatment of iron-deficiency anemia in childhood. Pediatr Hematol Oncol. 2004; 21:521–534.
Article45. Liu CP, Liu ZY, Liu JP, Kang Y, Mao CS, Shang J. Diagnostic value of common inflammatory markers on fever of unknown origin. Jpn J Infect Dis. 2016; 69:378–383.
Article46. Wu AC, Lesperance L, Bernstein H. Screening for iron deficiency. Pediatr Rev. 2002; 23:171–178.
Article47. Parodi E, Giraudo MT, Ricceri F, Aurucci ML, Mazzone R, Ramenghi U. Absolute reticulocyte count and reticulocyte hemoglobin content as predictors of early response to exclusive oral iron in children with iron deficiency anemia. Anemia. 2016; 2016:7345835.
Article48. Constantino BT. The red cell histogram and the dimorphic red cell population. Lab Med. 2011; 42:300–308.
Article49. Taylor S, Rampton D. Treatment of iron deficiency anemia: practical considerations. Pol Arch Med Wewn. 2015; 125:452–460.
Article50. Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol. 2002; 117:802–808.
Article51. Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012; 2:a011668.
Article52. Wang CY, Meynard D, Lin HY. The role of TMPRSS6/matriptase-2 in iron regulation and anemia. Front Pharmacol. 2014; 5:114.
Article53. Jaspers A, Caers J, Le Gac G, Ferec C, Beguin Y, Fillet G. A novel mutation in the CUB sequence of matriptase-2 (TMPRSS6) is implicated in iron-resistant iron deficiency anaemia (IRIDA). Br J Haematol. 2013; 160:564–565.
Article54. Wahedi M, Wortham AM, Kleven MD, et al. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J Biol Chem. 2017; 292:18354–18371.
Article55. Zhao N, Zhang AS, Enns CA. Iron regulation by hepcidin. J Clin Invest. 2013; 123:2337–2343.
Article56. McDonald CJ, Ostini L, Bennett N, et al. Functional analysis of matriptase-2 mutations and domains: insights into the molecular basis of iron-refractory iron deficiency anemia. Am J Physiol Cell Physiol. 2015; 308:C539–C547.
Article57. Azad SM, Kapoor R, Bannerji R, Ray J, Mitra M. Celiac disease masquerading as refractory iron deficiency anemia. Int J Contemp Pediatr. 2017; 4:672–673.
Article58. Ofei KT. Nutrient intakes and vitamin supplements in early pregnancy in relation to maternal age and body mass index in Umeå. Umeå, Sweden: Umeå International School of Public Health;2009.59. Schwartz WJ 3rd, Thurnau GR. Iron deficiency anemia in pregnancy. Clin Obstet Gynecol. 1995; 38:443–454.
Article60. Erslev AJ, Besarab A. Erythropoietin in the pathogenesis and treatment of the anemia of chronic renal failure. Kidney Int. 1997; 51:622–630.
Article61. Singh AK, Coyne DW, Shapiro W, Rizkala AR. DRIVE Study Group. Predictors of the response to treatment in anemic hemodialysis patients with high serum ferritin and low transferrin saturation. Kidney Int. 2007; 71:1163–1171.
Article62. Beguin Y, Loo M, R'Zik S, et al. Early prediction of response to recombinant human erythropoietin in patients with the anemia of renal failure by serum transferrin receptor and fibrinogen. Blood. 1993; 82:2010–2016.
Article63. Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis. 1996; 27:234–238.
Article64. Van Wyck DB, Stivelman JC, Ruiz J, Kirlin LF, Katz MA, Ogden DA. Iron status in patients receiving erythropoietin for dialysis-associated anemia. Kidney Int. 1989; 35:712–716.
Article65. Sarzynski E, Puttarajappa C, Xie Y, Grover M, Laird-Fick H. Association between proton pump inhibitor use and anemia: a retrospective cohort study. Dig Dis Sci. 2011; 56:2349–2353.
Article66. Glahn RP, Wortley GM, South PK, Miller DD. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: studies using an in vitro digestion/Caco-2 cell model. J Agric Food Chem. 2002; 50:390–395.
Article67. Sandberg AS, Brune M, Carlsson NG, Hallberg L, Skoglund E, Rossander-Hulthén L. Inositol phosphates with different numbers of phosphate groups influence iron absorption in humans. Am J Clin Nutr. 1999; 70:240–246.
Article68. Croft RF, Streeter AM, O'Neill BJ. Red cell indices in megaloblastosis and iron deficiency. Pathology. 1974; 6:107–117.
Article69. Walters MC, Abelson HT. Interpretation of the complete blood count. Pediatr Clin North Am. 1996; 43:599–622.
Article70. Spivak JL. Masked megaloblastic anemia. Arch Intern Med. 1982; 142:2111–2114.
Article71. Remacha AF, Sardà MP, Canals C, et al. Combined cobalamin and iron deficiency anemia: a diagnostic approach using a model based on age and homocysteine assessment. Ann Hematol. 2013; 92:527–531.
Article72. Green R. Folate, cobalamin, and megaloblastic anemias. In : Kaushansky K, Lichtman MA, Prchal JT, editors. Williams hematology. 9th ed. New York, NY: McGraw-Hill;2017. p. 596–641.73. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016; 91:31–38.
Article74. Schröder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease-a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005; 100:2503–2509.
Article75. Fishbane S, Block GA, Loram L, et al. Effects of ferric citrate in patients with nondialysis-dependent ckd and iron deficiency anemia. J Am Soc Nephrol. 2017; 28:1851–1858.
Article76. Ekanayake D, Roddick C, Powell LW. Recent advances in hemochromatosis: a 2015 update : a summary of proceedings of the 2014 conference held under the auspices of hemochromatosis Australia. Hepatol Int. 2015; 9:174–182.77. Harrison SA, Bacon BR. Hereditary hemochromatosis: update for 2003. J Hepatol. 2003; 38:Suppl 1. S14–S23.
Article78. Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014; 123:326–333.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple High-affinity Iron-uptake Systems of Vibrio vulnificus
- Effect of Iron-Uptake System on the Growth of Staphylococcus aureus according to the Iron Concentration and Oxygen Tension
- Altered Biodistribution of Gallium-67 in a Patient with Multiple Factors Influencing Iron-transport Protein Saturation
- Basic Understanding of Iron Metabolism
- Six Cases of Iron Containing Plasma Cells