J Rheum Dis.
2022 Apr;29(2):79-88. 10.4078/jrd.2022.29.2.79.
Assessment on Treatments With Conventional Synthetic Disease-modifying Drugs Before Initiating Biologics in Patients With Rheumatoid Arthritis in Korea: A Populationbased Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 2Division of Rheumatology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 3Division of Rheumatology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 4Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 5Division of Rheumatology, Department of Internal Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- KMID: 2527547
- DOI: http://doi.org/10.4078/jrd.2022.29.2.79
Abstract
Objective
To assess pre-biologic treatments with conventional synthetic disease-modifying drugs (csDMARDs) prior to biologics initiation among patients with rheumatoid arthritis (RA).
Methods
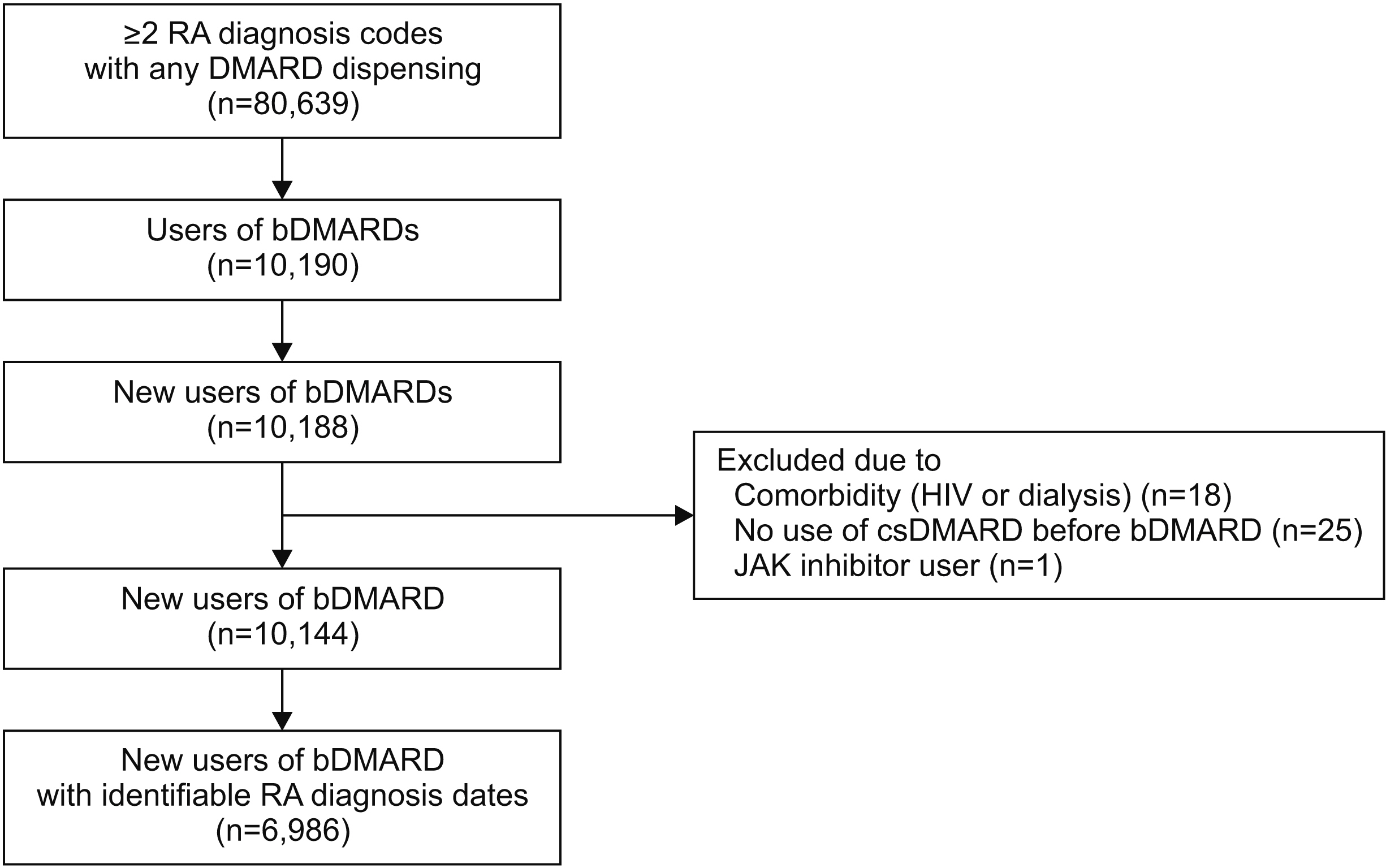
Using Korea National Health Insurance database, we examined pre-biologic treatments of RA patients on the following four items: whether 1) initial methotrexate (MTX) therapy was given, 2) MTX dose was escalated up to ≥15 mg/week within 1-year post-diagnosis, 3) prednisone-equivalent glucocorticoid was used at a dose of ≤7.5 mg/day, and 4) glucocorticoid was discontinued within 6 months of treatment. Multivariable logistic regressions identified predictors of items 2) and 4) fulfillment.
Results
Among 6,986 biologics initiators with RA, 54.9% used MTX as the 1st csDMARD. Within 1-year post-diagnosis, 85.2% used MTX with half of them achieving a dose of ≥15 mg/week. The majority (75.2%) of patients used glucocorticoids initially and 64.5% were still on glucocorticoids at 6 months, mostly at a dose of ≤7.5 mg/day. csDMARD combination was observed in 85.7%. Item 2) fulfillment was associated with males, younger age, glucocorticoid, combination therapy, cyclo-oxygenase-2 inhibitors, and viral hepatitis. Item 4) fulfillment was associated with males, MTX dose of ≥15 mg/week, combination therapy, viral hepatitis, and hospitalizations.
Conclusion
RA patients in Korea were predominantly treated with MTX-based csDMARD combination plus glucocorticoids before initiating biologics, without sufficient MTX dose escalation or glucocorticoid discontinuation. Items 2) and 4) fulfillments were associated with patient age and gender, concomitant treatments, and comorbidities.
Keyword
Figure
Reference
-
1. Bortoluzzi A, Valesini G, D'Angelo S, Frediani B, Bazzichi L, Afeltra A, et al. 2018; Immediate treatment with tumour necrosis factor inhibitors in synthetic disease-modifying anti-rheumatic drugs-naïve patients with rheumatoid arthritis: results of a modified Italian Expert Consensus. Rheumatology (Oxford). 57(57 Suppl 7):vii32–41. DOI: 10.1093/rheumatology/key076. PMID: 30289538.
Article2. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012; 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 64:625–39. DOI: 10.1002/acr.21641. PMID: 22473917. PMCID: PMC4081542.
Article3. Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. 2010; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 69:964–75. DOI: 10.1136/ard.2009.126532. PMID: 20444750. PMCID: PMC2935329.4. Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. 2014; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 73:492–509. DOI: 10.1136/annrheumdis-2013-204573. PMID: 24161836. PMCID: PMC3933074.5. Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. 2007; Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 66:1443–9. DOI: 10.1136/ard.2007.071092. PMID: 17519278. PMCID: PMC2111604.
Article6. Visser K, van der Heijde D. 2009; Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis. 68:1094–9. DOI: 10.1136/ard.2008.092668. PMID: 19033290. PMCID: PMC2689521.
Article7. Gaujoux-Viala C, Rincheval N, Dougados M, Combe B, Fautrel B. 2017; Optimal methotrexate dose is associated with better clinical outcomes than non-optimal dose in daily practice: results from the ESPOIR early arthritis cohort. Ann Rheum Dis. 76:2054–60. DOI: 10.1136/annrheumdis-2017-211268. PMID: 28866645.
Article8. Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, et al. 2009; Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 68:1086–93. DOI: 10.1136/ard.2008.094474. PMID: 19033291. PMCID: PMC2689523.
Article9. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. 2011; Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 13:R32. DOI: 10.1186/ar3260. PMID: 21345216. PMCID: PMC3241376.
Article10. van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. 2012; Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet. 379:1712–20. DOI: 10.1016/S0140-6736(12)60027-0.
Article11. de Jong PH, Hazes JM, Barendregt PJ, Huisman M, van Zeben D, van der Lubbe PA, et al. 2013; Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann Rheum Dis. 72:72–8. DOI: 10.1136/annrheumdis-2011-201162. PMID: 22679301.
Article12. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. 2012; A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 64:2824–35. DOI: 10.1002/art.34498. PMID: 22508468. PMCID: PMC4036119.
Article13. O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. 2013; Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med. 369:307–18. DOI: 10.1056/NEJMoa1303006. PMID: 23755969.14. Needham DM, Scales DC, Laupacis A, Pronovost PJ. 2005; A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 20:12–9. DOI: 10.1016/j.jcrc.2004.09.007. PMID: 16015512.
Article15. Cochran WG. 1954; Some methods for strengthening the common χ2 tests. Biometrics. 10:417–51. DOI: 10.2307/3001616.16. Armitage P. 1955; Tests for linear trends in proportions and frequencies. Biometrics. 11:375–86. DOI: 10.2307/3001775.
Article17. Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, et al. 2001; Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum. 44:1984–92. DOI: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B.18. Furst DE, Saag K, Fleischmann MR, Sherrer Y, Block JA, Schnitzer T, et al. 2002; Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with methotrexate: a six-month, double-blind, randomized, dose-ranging study. Arthritis Rheum. 46:2020–8. DOI: 10.1002/art.10427. PMID: 12209503.
Article19. Desai RJ, Solomon DH, Liu J, Kim SC. 2015; Secular trends in use of disease modifying anti-rheumatic drugs for the treatment of rheumatoid arthritis in the United States. Arthritis Rheumatol. 67(Suppl 10):2725. Available.20. Kaló Z, Vokó Z, Östör A, Clifton-Brown E, Vasilescu R, Battersby A, et al. 2017; Patient access to reimbursed biological disease-modifying antirheumatic drugs in the European region. J Mark Access Health Policy. 5:1345580. DOI: 10.1080/20016689.2017.1345580. PMID: 28740623. PMCID: PMC5508389.
Article21. Curtis JR, Zhang J, Xie F, Beukelman T, Chen L, Fernandes J, et al. 2014; Use of oral and subcutaneous methotrexate in rheumatoid arthritis patients in the United States. Arthritis Care Res (Hoboken). 66:1604–11. DOI: 10.1002/acr.22383. PMID: 24942466.
Article22. Schnabel A, Reinhold-Keller E, Willmann V, Gross WL. 1994; Tolerability of methotrexate starting with 15 or 25 mg/week for rheumatoid arthritis. Rheumatol Int. 14:33–8. DOI: 10.1007/BF00302669. PMID: 7939138.
Article23. Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A, et al. 2010; Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 69:43–7. DOI: 10.1136/ard.2008.101378. PMID: 19147616. PMCID: PMC2794929.
Article24. Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, Colls J, et al. 2020; Adverse effects of low-dose methotrexate: a randomized trial. Ann Intern Med. 172:369–80. DOI: 10.7326/M19-3369. PMID: 32066146. PMCID: PMC7229518.25. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. 2017; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 76:960–77. DOI: 10.1136/annrheumdis-2016-210715. PMID: 28264816.26. Kameda H, Fujii T, Nakajima A, Koike R, Sagawa A, Kanbe K, et al. 2019; Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol. 29:31–40. DOI: 10.1080/14397595.2018.1472358. PMID: 29718746.
Article27. Svensson B, Boonen A, Albertsson K, van der Heijde D, Keller C, Hafström I. 2005; Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum. 52:3360–70. DOI: 10.1002/art.21298. PMID: 16255010.
Article28. Tengstrand B, Larsson E, Klareskog L, Hafström I. 2007; Randomized withdrawal of long-term prednisolone treatment in rheumatoid arthritis: effects on inflammation and bone mineral density. Scand J Rheumatol. 36:351–8. DOI: 10.1080/03009740701394021. PMID: 17963164.
Article29. Smolen JS, Landewé RBM, van der Heijde D. 2017; Response to: '2016 update of the EULAR recommendations for the management of rheumatoid arthritis: no utopia for patients in low/middle-income countries?' by Misra et al. Ann Rheum Dis. 76:e48. DOI: 10.1136/annrheumdis-2017-211455. PMID: 28478402.30. Singer O, Gibofsky A. 2011; Methotrexate versus leflunomide in rheumatoid arthritis: what is new in 2011? Curr Opin Rheumatol. 23:288–92. DOI: 10.1097/BOR.0b013e328344f2e4. PMID: 21378570.
Article31. Nakajima A, Inoue E, Taniguchi A, Momohara S, Yamanaka H. 2016; Effectiveness of tacrolimus in comparison with methotrexate or biologics in propensity score-matched patients with rheumatoid arthritis. Mod Rheumatol. 26:836–43. DOI: 10.3109/14397595.2016.1160969. PMID: 26934454.
Article32. Park EH, Shin A, Ha YJ, Lee YJ, Lee EB, Song YW, et al. 2021; Risk factors associated with initiation of a biologic disease modifying anti-rheumatic drug in patients with rheumatoid arthritis: a nested case-control study on 34,925 patients. Joint Bone Spine. 88:105057. DOI: 10.1016/j.jbspin.2020.07.006. PMID: 32711117.
Article33. Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A. Equity in Clinical Eligibility Criteria for RA treatment Working Group. 2014; Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country's wealth? Ann Rheum Dis. 73:2010–21. DOI: 10.1136/annrheumdis-2013-203819. PMID: 23940213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Positive Effects of Biologics on Osteoporosis in Rheumatoid Arthritis
- Use of Disease-modifying Antirheumatic Drugs After Cancer Diagnosis in Rheumatoid Arthritis Patients
- Medical Treatment of Rheumatoid Arthritis
- Medical treatment of rheumatoid arthritis (I): Nonsteroidal anti-inflammatory drugs, disease modifying antirheumatic drugs and glucocorticoids
- Cementless Total Knee Arthroplasty and Effects of Disease Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis