Lab Med Online.
2021 Oct;11(4):235-244. 10.47429/lmo.2021.11.4.235.
Comparison of Three Automated Calcitonin Immunoassays for Evaluating the Equivalence Near the Clinical Decision Point
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2526084
- DOI: http://doi.org/10.47429/lmo.2021.11.4.235
Abstract
- Background
To date, only a few studies have focused on the standardization of the calcitonin assay, despite the fact that a lack of standardization of assays can lead to discrepancy in results. Here, we analyzed the concordance in serum calcitonin test results using three different assays.
Methods
The serum calcitonin levels in 104 residual specimens collected between January and February 2020 were measured using three different systems. The Spearman’s rank correlation coefficients and the slopes and y-intercepts were assessed to derive all possible pairs of analyzers. The agreement of classification according to a clinically relevant cut-off was also evaluated using Cohen’s kappa coefficient.
Results
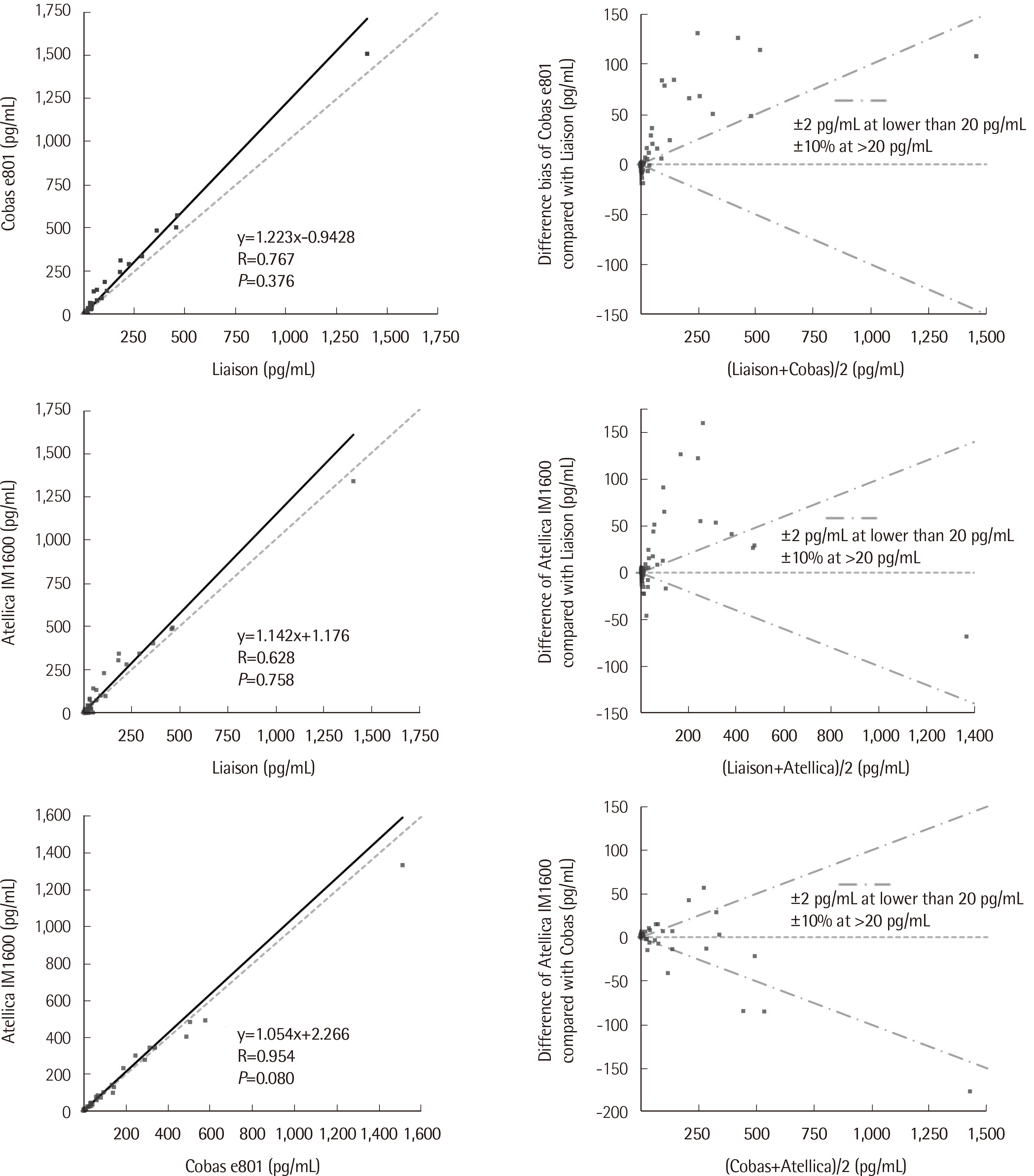
The correlation coefficients for Cobas e801 versus LIAISON, Atellica IM-1600 versus LIAISON, and Cobas e801 versus Atellica IM-1600 were 0.77, 0.63, and 0.95, respectively. The kappa agreement of classification at a cut-off of 10 pg/mL was 0.81, 0.81, and 0.91, respectively. However, after excluding the data points for concentrations > 20 pg/mL, the correlation coefficients decreased to 0.39, 0.22, and 0.90, respectively.
Conclusions
Despite acceptable correlations for the full analytical measuring range, we observed limited correlations at low concentrations, especially around the clinical decision threshold. Therefore, continuous joint efforts by all stakeholders are essential for standardizing calcitonin assays.
Keyword
Figure
Reference
-
1. Copp DH, Cameron EC, Cheney BA, Davidson AG, Henze KG. 1962; Evidence for calcitonin--a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 70:638–49. DOI: 10.1210/endo-70-5-638. PMID: 13881211.
Article2. Hirsch PF, Voelkel EF, Munson PL. 1964; Thyrocalcitonin: hypocalcemic hypophosphatemic principle of the thyroid gland. Science. 146:412–3. DOI: 10.1126/science.146.3642.412. PMID: 14186470.
Article3. Austin LA, Heath H 3rd. 1981; Calcitonin: physiology and pathophysiology. N Engl J Med. 304:269–78. DOI: 10.1056/NEJM198101293040505. PMID: 7003392.4. Machens A, Hoffmann F, Sekulla C, Dralle H. 2009; Importance of gender-specific calcitonin thresholds in screening for occult sporadic medullary thyroid cancer. Endocr Relat Cancer. 16:1291–8. DOI: 10.1677/ERC-09-0136. PMID: 19726541.
Article5. Cavalier E, Carlisi A, Bekaert AC, Rousselle O, Chapelle JP, Delanaye P. 2011; Analytical validation of the Liaison Calcitonin_II-Gen (DiaSorin). Clin Chem Lab Med. 49:271–5. DOI: 10.1515/CCLM.2011.036. PMID: 21083442.
Article6. Guilloteau D, Perdrisot R, Calmettes C, Baulieu JL, Lecomte P, Kaphan G, et al. 1990; Diagnosis of medullary carcinoma of the thyroid (MCT) by calcitonin assay using monoclonal antibodies: criteria for the pentagastrin stimulation test in hereditary MCT. J Clin Endocrinol Metab. 71:1064–7. DOI: 10.1210/jcem-71-4-1064. PMID: 2401708.
Article7. Tobler PH, Tschopp FA, Dambacher MA, Born W, Fischer JA. 1983; Identification and characterization of calcitonin forms in plasma and urine of normal subjects and medullary carcinoma patients. J Clin Endocrinol Metab. 57:749–54. DOI: 10.1210/jcem-57-4-749. PMID: 6885964.
Article8. Cheung K, Roman SA, Wang TS, Walker HD, Sosa JA. 2008; Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 93:2173–80. DOI: 10.1210/jc.2007-2496. PMID: 18364376.
Article9. Kahaly GJ, Algeciras-Schimnich A, Davis TE, Diana T, Feldkamp J, Karger S, et al. 2017; United States and European multicenter prospective study for the analytical performance and clinical validation of a novel sensitive fully automated immunoassay for calcitonin. Clin Chem. 63:1489–96. DOI: 10.1373/clinchem.2016.270009. PMID: 28687633.
Article10. Allelein S, Ehlers M, Morneau C, Schwartz K, Goretzki PE, Seppel T, et al. 2018; Measurement of basal serum calcitonin for the diagnosis of medullary thyroid cancer. Horm Metab Res. 50:23–8. DOI: 10.1055/s-0043-122237. PMID: 29169190.
Article11. Rink T, Truong PN, Schroth HJ, Diener J, Zimny M, Grünwald F. 2009; Calculation and validation of a plasma calcitonin limit for early detection of medullary thyroid carcinoma in nodular thyroid disease. Thyroid. 19:327–32. DOI: 10.1089/thy.2008.0102. PMID: 19355822.
Article12. Engelbach M, Gorges R, Forst T, Pfutzner A, Dawood R, Heerdt S, et al. 2000; Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab. 85:1890–4. DOI: 10.1210/jcem.85.5.6601. PMID: 10843170.
Article13. Wion-Barbot N, Schuffenecker I, Niccoli P, Conte-Devolx B, Lecomte P, Houdent C, et al. 1997; Results of the calcitonin stimulation test in normal volunteers compared with genetically unaffected members of MEN 2A and familial medullary thyroid carcinoma families. Ann Endocrinol (Paris). 58:302–8. PMID: 9436479.14. Conte-Devolx B, Schuffenecker I, Niccoli P, Maes B, Boneu A, Barbot N, et al. 1997; Multiple endocrine neoplasia type 2: management of patients and subjects at risk. French Study Group on Calcitonin-Secreting Tumors (GETC). Horm Res. 47:221–6. DOI: 10.1159/000185467. PMID: 9167955.15. Vierhapper H, Niederle B, Bieglmayer C, Kaserer K, Baumgartner-Parzer S. 2005; Early diagnosis and curative therapy of medullary thyroid carcinoma by routine measurement of serum calcitonin in patients with thyroid disorders. Thyroid. 15:1267–72. DOI: 10.1089/thy.2005.15.1267. PMID: 16356091.
Article16. Karges W, Dralle H, Raue F, Mann K, Reiners C, Grussendorf M, et al. 2004; Calcitonin measurement to detect medullary thyroid carcinoma in nodular goiter: German evidence-based consensus recommendation. Exp Clin Endocrinol Diabetes. 112:52–8. DOI: 10.1055/s-2004-815727. PMID: 14758572.
Article17. Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, et al. 2004; Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 89:163–8. DOI: 10.1210/jc.2003-030550. PMID: 14715844.
Article18. Ozgen AG, Hamulu F, Bayraktar F, Yilmaz C, Tuzun M, Yetkin E, et al. 1999; Evaluation of routine basal serum calcitonin measurement for early diagnosis of medullary thyroid carcinoma in seven hundred seventy-three patients with nodular goiter. Thyroid. 9:579–82. DOI: 10.1089/thy.1999.9.579. PMID: 10411120.
Article19. Cavalier E, Carlisi A, Chapelle JP, Delanaye P. 2008; Analytical quality of calcitonin determination and its effect on the adequacy of screening for medullary carcinoma of the thyroid. Clin Chem. 54:929–30. DOI: 10.1373/clinchem.2007.100636. PMID: 18443183.
Article20. Schiettecatte J, Strasser O, Anckaert E, Smitz J. 2016; Performance evaluation of an automated electrochemiluminescent calcitonin (CT) immunoassay in diagnosis of medullary thyroid carcinoma. Clin Biochem. 49:929–31. DOI: 10.1016/j.clinbiochem.2016.05.006. PMID: 27182955.
Article21. Motté P, Vauzelle P, Gardet P, Ghillani P, Caillou B, Parmentier C, et al. 1988; Construction and clinical validation of a sensitive and specific assay for serum mature calcitonin using monoclonal anti-peptide antibodies. Clin Chim Acta. 174:35–54. DOI: 10.1016/0009-8981(88)90365-8. PMID: 2454766.
Article22. Leclerc L, Vantyghem MC, Fourrier F, Proye C, Wemeau JL. d'Herbomez M. 2001; Clinical evaluation of a new sensitive calcitonin assay: study of specificity. Clin Chim Acta. 311:149–55. DOI: 10.1016/S0009-8981(01)00582-4. PMID: 11566174.23. Bieglmayer C, Vierhapper H, Dudczak R, Niederle B. 2007; Measurement of calcitonin by immunoassay analyzers. Clin Chem Lab Med. 45:662–6. DOI: 10.1515/CCLM.2007.124. PMID: 17484631.
Article24. Clinical and Laboratory Standards Institute. 2018. Measurement procedure comparison and bias estimation using patient samples; Approved guideline-Third edition. EP09c-ED3. Clinical and Laboratory Standards Institute;EP09c-ED3. Wayne, PA:25. McPherson RA, Pincus MR, editors. 2017. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. Elsevier;St. Louis, MO:26. The Royal College of Pathologists of Australasia Quality Assurance Programs. Allowable limits of performance. (Programs, Analytes and Allowable Limits of Performance). http://www.rcpaqap.com.au/docs/2014/chempath/ALP.pdf. Updated on Aug 2014.27. National Institute for Biological Standards and Control. WHO International Standard, Calcitonin, Human, NIBSC code: 89/620, Instructions for use, (Version 5.0, Dated 28/03/2013). https://www.nibsc.org/documents/ifu/89-620.pdf. Last accessed on Feb 2021.28. Herrmann BL, Schmid KW, Goerges R, Kemen M, Mann K. 2010; Calcitonin screening and pentagastrin testing: predictive value for the diagnosis of medullary carcinoma in nodular thyroid disease. Eur J Endocrinol. 162:1141–5. DOI: 10.1530/EJE-10-0111. PMID: 20332126.
Article29. Ramachandran R, Benfield P, Dhillo WS, White S, Chapman R, Meeran K, et al. 2009; Need for revision of diagnostic limits for medullary thyroid carcinoma with a new immunochemiluminometric calcitonin assay. Clin Chem. 55:2225–6. DOI: 10.1373/clinchem.2009.129361. PMID: 19833839.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concordance of Three Automated Procalcitonin Immunoassays at Medical Decision Points
- Comparison of 3 Automated Immunoassays for Hepatitis B Surface Antigen
- Equivalence Margin of the Biosimilar Product
- Effect of Antiandrogen on Calcitonin Gene-related Peptide mRNA Expression ofthe Rat Cremaster Nucleus
- Medullary Thyroid Carcinoma with Normal Calcitonin Level