J Korean Soc Clin Pharmacol Ther.
2012 Jun;20(1):17-33.
Equivalence Margin of the Biosimilar Product
- Affiliations
-
- 118515 Fontana Lane, Gaithersburg, MD 20879, USA. leehwd@gmail.com
Abstract
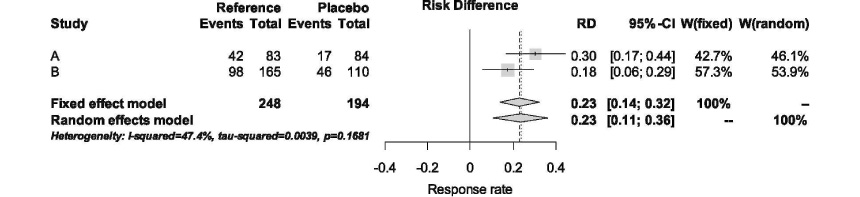
- The equivalence margin is the largest difference that is clinically acceptable between the test (or experimental) drug and the active control (or reference) drug. This paper discusses the scientific principles, along with the regulatory issues, that need to be addressed when determining the equivalence margin for the biosimilar product. The concept of assay sensitivity is introduced, and the ways to ensure assay sensitivity in the equivalence trial are emphasized. A hypothetical example is presented to show how an equivalence margin is determined. The regulatory agency should carefully assess if the equivalence margin of the biosimilar product was determined using a scientifically valid and clinically relevant approach, not subject to selection bias. This is important because the consumer risk of erroneously declaring equivalence when in fact it is not must be controlled conservatively low in the approval of any biosimilar products.
MeSH Terms
Figure
Reference
-
1. McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012. 91(3):405–417.
Article2. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008. 19(3):411–419.
Article3. Njue C. Statistical considerations for confirmatory clinical trials for similar biotherapeutic products. Biologicals. 2011. 39(5):266–269.
Article4. Korea Food & Drug Administration. National Institute of Food and Drug Safety Evaluation. Guidelines on the Evaluation of Biosimilar Products. 2009. Seoul (Korea):5. World Health Organization. Guidelines on evaluation of similar biotherapeutic products (SBPs). 2012.6. Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). Guidance for industry: Scientific considerations in demonstrating biosimilarity to a reference product (Draft). 2012.7. European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies (EMA/CHMP/BMWP/403543/2010). last visited on Feb 13 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099361.pdf. 10/2010, 2010. [Online].8. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). Guidance for Industry: Non-inferiority clinical trials (Draft). 2010. 03.9. Greene CJ, Morland LA, Durkalski VL, Frueh BC. Noninferiority and equivalence designs: issues and implications for mental health research. J Trauma Stress. 2008. 21(5):433–439.
Article10. Hwang IK, Morikawa T. Design Issues in Noninferiority/Equivalence Trials. Drug Inf J. 1999. 33(4):1205–1218.
Article11. United States Government Accountability Office. New drug approval: FDA's consideration of evidence from certain clinical trials. 2010. Washington, DC, USA: United States Government Accountability Office.12. Committee for Medicinal Products for Human Use (CHMP). Guideline on the Choice of the Non-inferiority margin. EMEA/CPMP/EWP/2158/99. 2005. 07. 27. London (UK):13. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009. 172(1):137–159.
Article14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. 7(3):177–188.
Article15. The Cochrane Collaboration. Higgins J, Green S, editors. 9.5.4 Incorporating heterogeneity into random-effects models. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. 2011. [updated March 2011].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Asian Physician's Perspectives on Biosimilars in Inflammatory Bowel Disease: Are We Ready to Use?
- Uptake of Biosimilars and Its Economic Implication for the Treatment of Patients with Rheumatoid Arthritis in Korea
- Current status of biosimilars in the treatment of inflammatory bowel diseases
- Efficacy and safety of the adalimumab biosimilar Exemptia as induction therapy in moderate-to-severe ulcerative colitis
- Knowledge and Viewpoints on Biosimilar Monoclonal Antibodies among Asian Physicians: Comparison with European Physicians