Lab Med Online.
2021 Jul;11(3):171-176. 10.47429/lmo.2021.11.3.171.
Re-evaluation of the Utility of the CH50 Test Using Liposome Immunoassay
- Affiliations
-
- 1Department of Laboratory Medicine, Hanyang University Seoul Hospital, Seoul, Korea
- 2Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2526062
- DOI: http://doi.org/10.47429/lmo.2021.11.3.171
Abstract
- Background
This study aimed to evaluate whether the 50% hemolytic complement (CH50) is a suitable screening test to investigate complement activity by comparing its liposomal immunoassay-based results with C3 and C4 values and determine if it meaningfully reflects changes in the complement system.
Methods
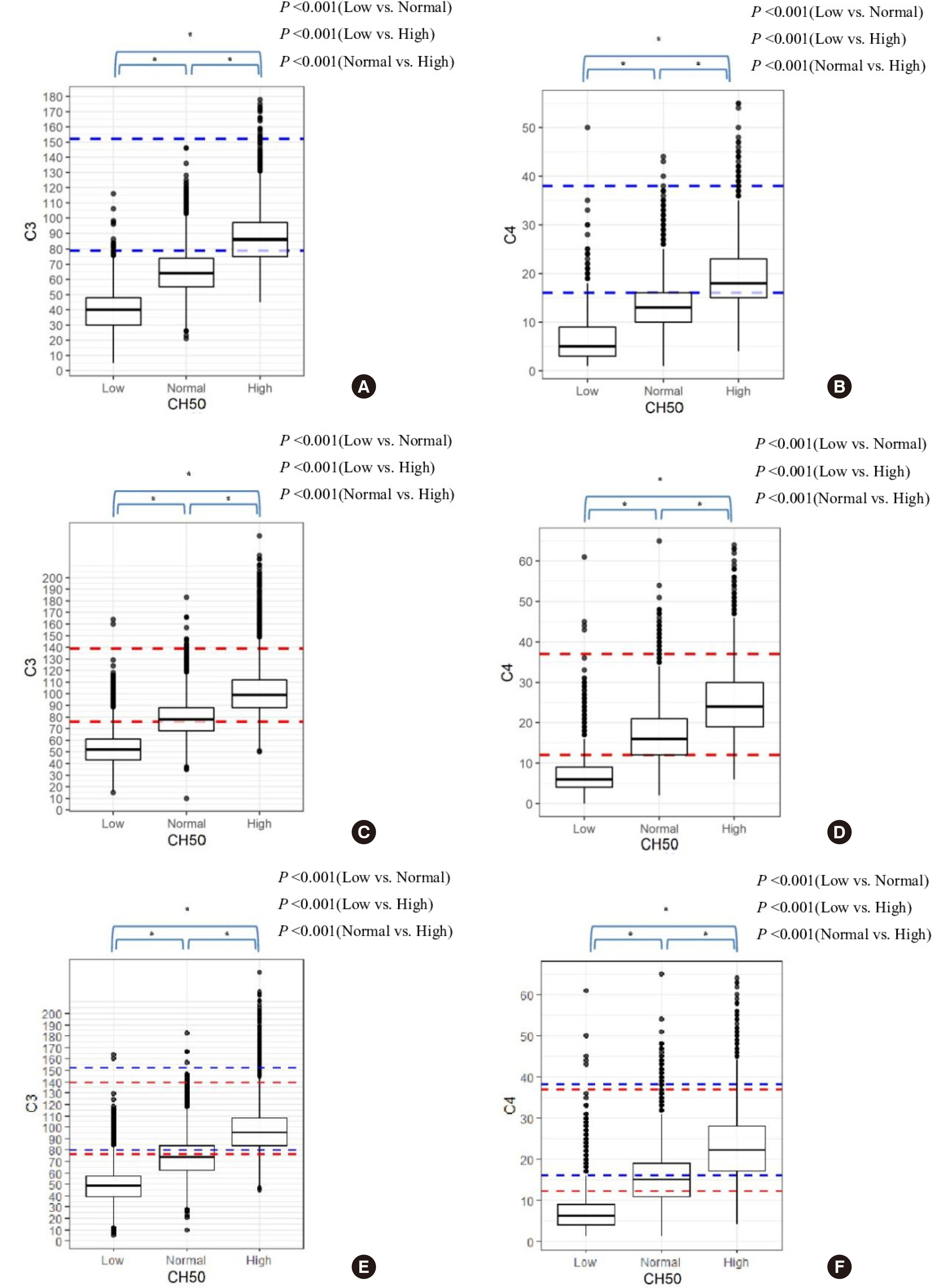
A retrospective study was conducted on 35,908 test samples that were simultaneously evaluated using C3, C4, and CH50 assays. Liposomal immunoassay was used for CH50 test, and rate nephelometry or immunoturbidimetry was used for C3 and C4 tests. The CH50, C3, and C4 results were divided into low, normal, and high groups for comparison and analysis. The distribution of C3 and C4 measurements according to the three different CH50 groups was analyzed.
Results
Of the 35,908 cases, the C3 and C4 results were decreased in 19,051 (53%) and 14,666 (41%) cases, respectively. However, CH50 results were decreased in 6,257 cases (17%), which were lower than those for C3 and C4. A statistically significant difference was observed in the distribution of C3 and C4 values among different CH50 groups (P < 0.001).
Conclusions
The CH50 test based on the liposome immunoassay does not sensitively reflect the decrease in C3 and C4. However, it tends to show a better decrease at low concentrations proportional to the decreased amount of C3 and C4. Hence, it can be useful for severe complement deficiency screening. Therefore, there are limitations with the use of the CH50 test alone for the screening of complement activity, and care should be taken during result interpretation.
Keyword
Figure
Reference
-
1. Henry JB, McPherson RA, editors. 2011. Henry's clinical diagnosis and management by laboratory methods. Elsevier/Saunders;Philadelphia, PA: p. 914–32.2. Ho A, Barr SG, Magder LS, Petri M. 2001; A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 44:2350–7. DOI: 10.1002/1529-0131(200110)44:10<2350::AID-ART398>3.0.CO;2-A.
Article3. Wen L, Atkinson JP, Giclas PC. 2004; Clinical and laboratory evaluation of complement deficiency. J Allergy Clin Immunol. 113:585–93. DOI: 10.1016/j.jaci.2004.02.003. PMID: 15100659.
Article4. Jaskowski TD, Martins TB, Litwin CM, Hill HR. 1999; Comparison of three different methods for measuring classical pathway complement activity. Clin Diagn Lab Immunol. 6:137–9. DOI: 10.1128/CDLI.6.1.137-139.1999. PMID: 9874678. PMCID: PMC95674.
Article5. Lee ZY, Jearn LH, Park IK, Kim TY. 2014; Alterations of complement C3 and C4 levels in delayed testing. Lab Med Online. 4:152–6. DOI: 10.3343/lmo.2014.4.3.152.
Article6. Yang S, McGookey M, Wang Y, Cataland SR, Wu HM. 2015; Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am J Clin Pathol. 143:558–65. DOI: 10.1309/AJCPXPD7ZQXNTIAL. PMID: 25780008.
Article7. Mollnes TE, Garred P, Bergseth G. 1988; Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol. 73:484–8.8. Maguire OC, Curry MP, O'Gorman P, Parfrey N, Hegarty J, Cunningham SK. 2001; In vitro cold activation of complement shown by an overestimation of total complement 4: a study in patients with hepatitis C virus infection. Ann Clin Biochem. 38:687–93. DOI: 10.1258/0004563011900902. PMID: 11732652.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Evaluation of Standard 50% Hemolytic Complement Assay Using In-house Reagents
- Total Haemolytic Complement Activity at Diagnosis as an Indicator of the Baseline Activity of Antineutrophil Cytoplasmic Antibody-associated Vasculitis
- The Effect of the Toxic Reaction of the Retina by Liposome-encapsulated Tobramycin in Normal Rabbits
- Evaluation of Dimension Hemoglobin A1c as an Emergency Outpatient Test
- Theranostics Based on Liposome: Looking Back and Forward