J Korean Assoc Oral Maxillofac Surg.
2021 Dec;47(6):454-464. 10.5125/jkaoms.2021.47.6.454.
Effects of the combination of bone morphogenetic protein-2 and nano-hydroxyapatite on the osseointegration of dental implants
- Affiliations
-
- 1Department of Dentistry, Oral and Maxillofacial Surgery, Seoul National University Dental Hospital, Seoul, Korea
- 2Department of Medical Biotechnology, College of Life Science and Biotechnology, Dongguk University, Seoul, Korea
- 3Department of Oral and Maxillofacial Surgery, School of Dentistry, Seoul National University, Seoul, Korea
- 4Dental Life Science Research Institute and Clinical Translational Research Center for Dental Science, Seoul National University Dental Hospital, Seoul, Korea
- KMID: 2524705
- DOI: http://doi.org/10.5125/jkaoms.2021.47.6.454
Abstract
Objectives
This study aimed to investigate the in vitro osteoinductivity of the combination of bone morphogenetic protein-2 (BMP-2) and nanohydroxyapatite (nHAp) and the in vivo effects of implants coated with nHAp/BMP-2.
Materials and Methods
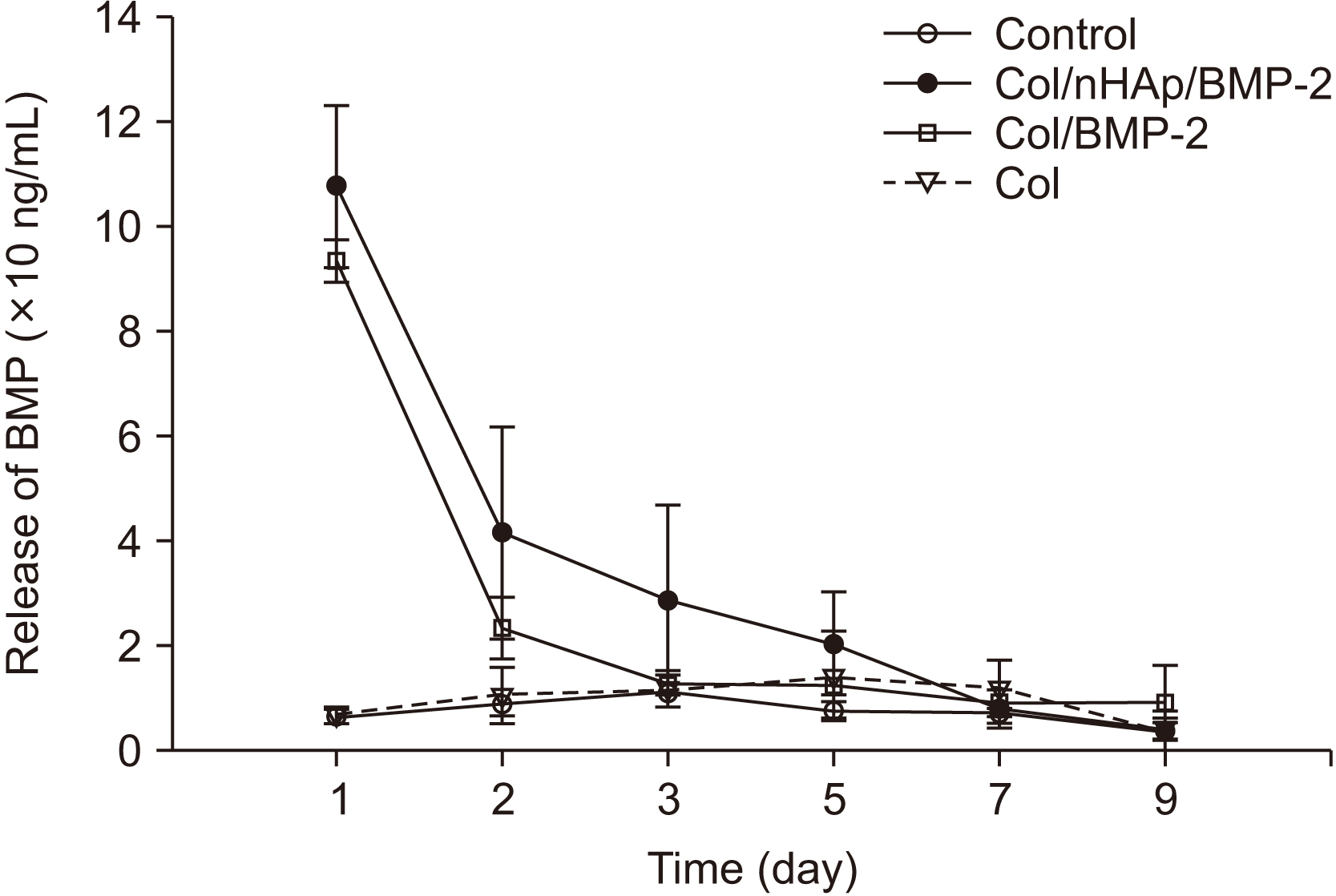
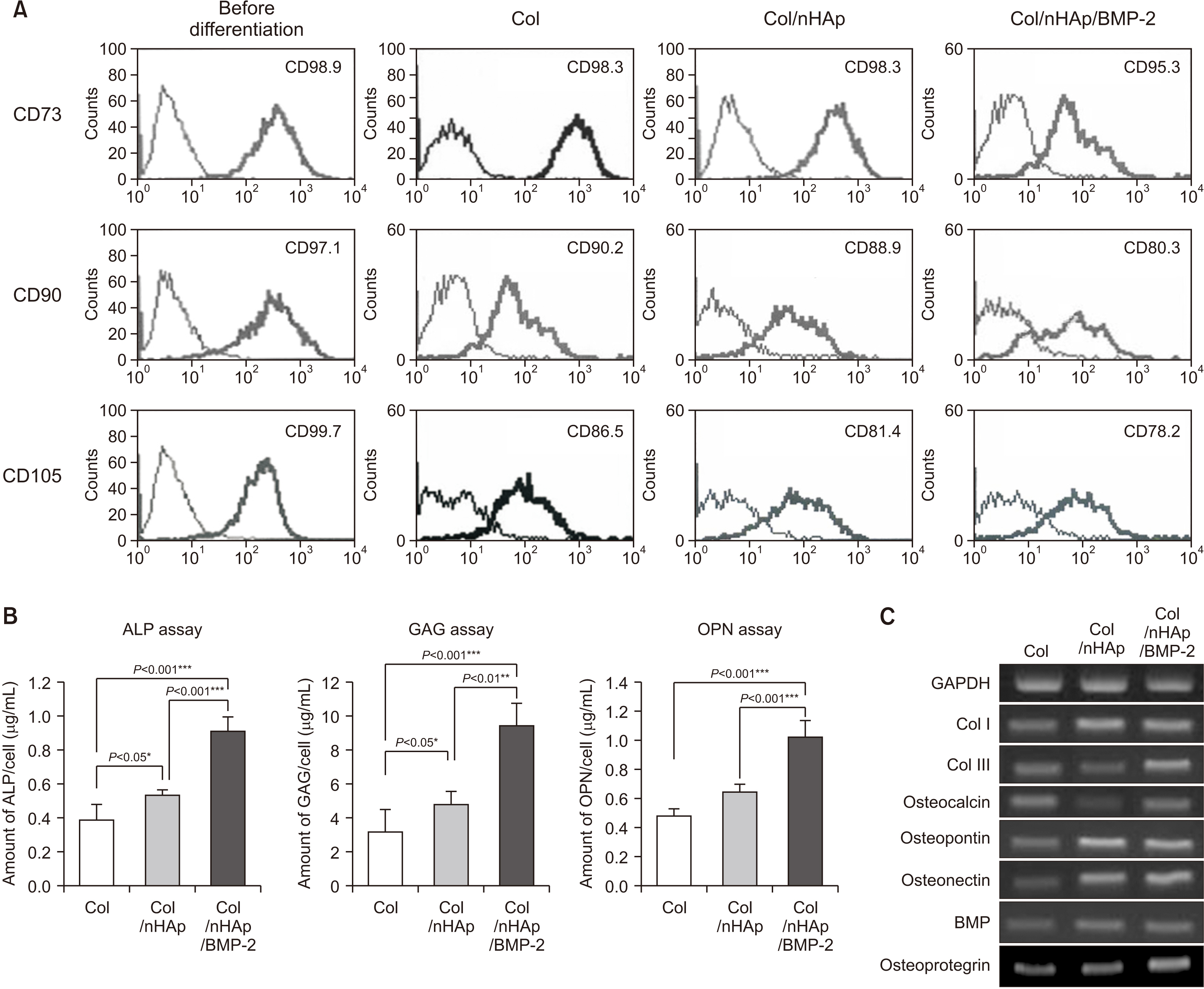
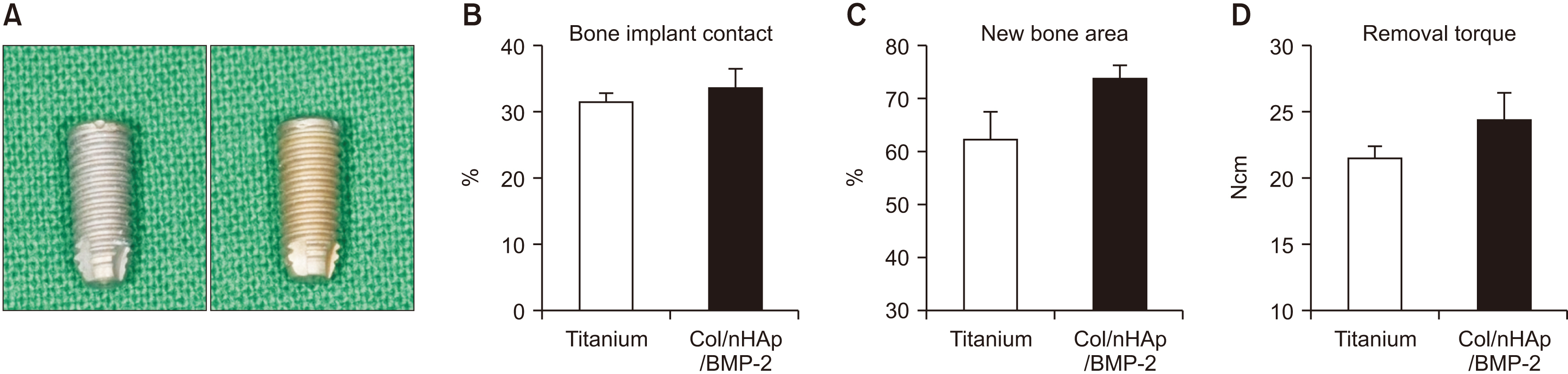
To evaluate the in vitro efficacy of nHAp/BMP-2 on bone formation, bone marrow-derived mesenchymal stem cells (BMMSCs) were seeded onto titanium disks coated with collagen (Col), Col/nHAp, or Col/nHAp/BMP-2. Protein levels were determined by a biochemical assay and reverse transcriptase-polymerase chain reaction. Stem cell differentiation was analyzed by flow cytometry. For in vivo studies with mice, Col, Col/nHAp, and Col/nHAp/BMP-2 were injected in subcutaneous pockets. Titanium implants or implants coated with Col/nHAp/BMP-2 were placed bilaterally on rabbit tibias and evaluated for 4 weeks.
Results
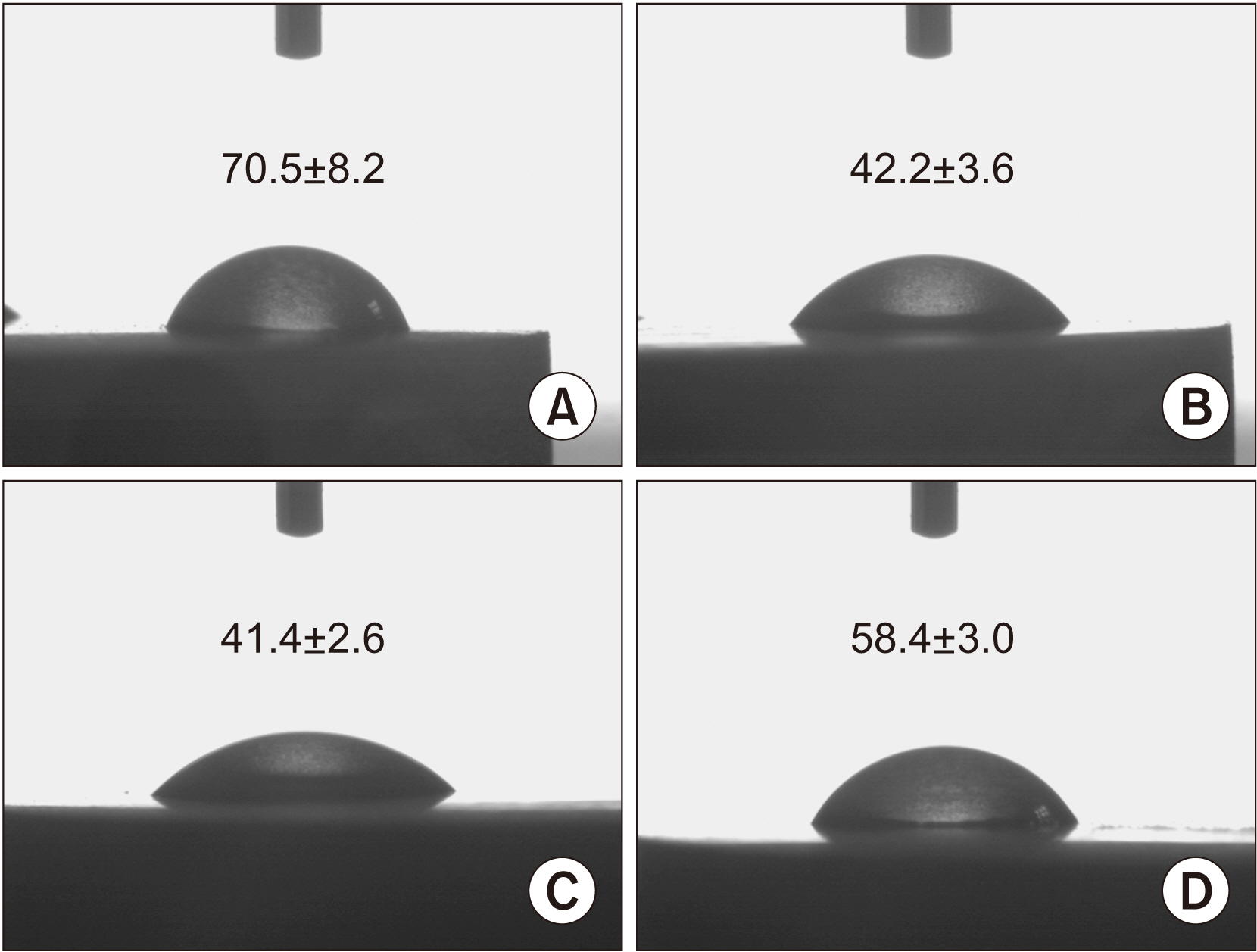
In the in vitro study, BM-MSCs on Col/nHAp/BMP-2 showed reduced levels of CD73, CD90, and CD105 and increased levels of glycos-aminoglycan, osteopontin, and alkaline phosphatase activity. After 4 weeks, the Col/nHAp/BMP-2 implant showed greater bone formation than the control (P=0.07), while no differences were observed in bone implant contact and removal torque.
Conclusion
These results suggest that a combination of BMP-2 and an nHAp carrier would activate osseointegration on dental implant surfaces.
Figure
Reference
-
References
1. Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G, et al. 2009; Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 88:579–96. https://doi.org/10.1002/jbm.b.31264. DOI: 10.1002/jbm.b.31264. PMID: 18973274.
Article2. Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. 2010; Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 28:198–206. https://doi.org/10.1016/j.tibtech.2009.12.003. DOI: 10.1016/j.tibtech.2009.12.003. PMID: 20116873.
Article3. Mendonça G, Mendonça DB, Aragão FJ, Cooper LF. 2008; Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 29:3822–35. https://doi.org/10.1016/j.biomaterials.2008.05.012. DOI: 10.1016/j.biomaterials.2008.05.012. PMID: 18617258.
Article4. Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Nishimura R, Wozney JM, et al. 2003; Long-term functional loading of dental implants in rhBMP-2 induced bone. A histologic study in the canine ridge augmentation model. Clin Oral Implants Res. 14:793–803. https://doi.org/10.1046/j.0905-7161.2003.clr140617.x. DOI: 10.1046/j.0905-7161.2003.clr140617.x. PMID: 15015957.
Article5. Park JC, Kim JC, Kim BK, Cho KS, Im GI, Kim BS, et al. 2012; Dose- and time-dependent effects of recombinant human bone morphogenetic protein-2 on the osteogenic and adipogenic potentials of alveolar bone-derived stromal cells. J Periodontal Res. 47:645–54. https://doi.org/10.1111/j.1600-0765.2012.01477.x. DOI: 10.1111/j.1600-0765.2012.01477.x. PMID: 22471302.
Article6. Lee JH, Yu CH, Yang JJ, Baek HR, Lee KM, Koo TY, et al. 2012; Comparative study of fusion rate induced by different dosages of Escherichia coli-derived recombinant human bone morphogenetic protein-2 using hydroxyapatite carrier. Spine J. 12:239–48. https://doi.org/10.1016/j.spinee.2012.01.013. DOI: 10.1016/j.spinee.2012.01.013. PMID: 22341396.
Article7. Fujimura K, Bessho K, Kusumoto K, Ogawa Y, Iizuka T. 1995; Experimental studies on bone inducing activity of composites of atelopeptide type I collagen as a carrier for ectopic osteoinduction by rhBMP-2. Biochem Biophys Res Commun. 208:316–22. https://doi.org/10.1006/bbrc.1995.1340. DOI: 10.1006/bbrc.1995.1340. PMID: 7887945.
Article8. Garrett MP, Kakarla UK, Porter RW, Sonntag VK. 2010; Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery. 66:1044–9. discussion 1049. https://doi.org/10.1227/01.NEU.0000369517.21018.F2. DOI: 10.1227/01.NEU.0000369517.21018.F2. PMID: 20495420.
Article9. Kokorina NA, Lewis JS Jr, Zakharkin SO, Krebsbach PH, Nussenbaum B. 2012; rhBMP-2 has adverse effects on human oral carcinoma cell lines in vivo. Laryngoscope. 122:95–102. https://doi.org/10.1002/lary.22345. DOI: 10.1002/lary.22345. PMID: 21997819. PMCID: PMC3895340.
Article10. Koyama N, Okubo Y, Nakao K, Osawa K, Bessho K. 2011; Experimental study of osteoinduction using a new material as a carrier for bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 49:314–8. https://doi.org/10.1016/j.bjoms.2010.05.010. DOI: 10.1016/j.bjoms.2010.05.010. PMID: 20554359.
Article11. Mindea SA, Shih P, Song JK. 2009; Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine (Phila Pa 1976). 34:1480–4. discussion 1485. https://doi.org/10.1097/BRS.0b013e3181a396a1. DOI: 10.1097/BRS.0b013e3181a396a1. PMID: 19525840.
Article12. Perri B, Cooper M, Lauryssen C, Anand N. 2007; Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 7:235–9. https://doi.org/10.1016/j.spinee.2006.04.010. DOI: 10.1016/j.spinee.2006.04.010. PMID: 17321975.
Article13. Smoljanovic T, Pecina M. 2006; Re: Burkus J K, Sandhu H S, Gornet M F. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 31:775–81. Spine (Phila Pa 1976) 2008;33:226. https://doi.org/10.1097/BRS.0b013e3181606894. DOI: 10.1097/BRS.0b013e3181606894. PMID: 18197115.
Article14. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. 2008; Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 8:1011–8. https://doi.org/10.1016/j.spinee.2007.06.014. DOI: 10.1016/j.spinee.2007.06.014. PMID: 18037352.
Article15. Wen B, Karl M, Pendrys D, Shafer D, Freilich M, Kuhn L. 2011; An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J Biomed Mater Res B Appl Biomater. 97:315–26. https://doi.org/10.1002/jbm.b.31817. DOI: 10.1002/jbm.b.31817. PMID: 21394902.
Article16. Zhang W, Tsurushima H, Oyane A, Yazaki Y, Sogo Y, Ito A, et al. 2011; BMP-2 gene-fibronectin-apatite composite layer enhances bone formation. J Biomed Sci. 18:62. https://doi.org/10.1186/1423-0127-18-62. DOI: 10.1186/1423-0127-18-62. PMID: 21859498. PMCID: PMC3175450.
Article17. Hulsart-Billström G, Hu Q, Bergman K, Jonsson KB, Åberg J, Tang R, et al. 2011; Calcium phosphates compounds in conjunction with hydrogel as carrier for BMP-2: a study on ectopic bone formation in rats. Acta Biomater. 7:3042–9. https://doi.org/10.1016/j.actbio.2011.04.021. DOI: 10.1016/j.actbio.2011.04.021. PMID: 21569871.
Article18. Li J, Li Y, Ma S, Gao Y, Zuo Y, Hu J. 2010; Enhancement of bone formation by BMP-7 transduced MSCs on biomimetic nano-hydroxyapatite/polyamide composite scaffolds in repair of mandibular defects. J Biomed Mater Res A. 95:973–81. https://doi.org/10.1002/jbm.a.32926. DOI: 10.1002/jbm.a.32926. PMID: 20845497.
Article19. Liu Y, Lu Y, Tian X, Cui G, Zhao Y, Yang Q, et al. 2009; Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials. 30:6276–85. https://doi.org/10.1016/j.biomaterials.2009.08.003. DOI: 10.1016/j.biomaterials.2009.08.003. PMID: 19683811.
Article20. Gomez B Jr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, et al. 1995; Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem. 41:1560–6. DOI: 10.1093/clinchem/41.11.1560.
Article21. Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, et al. 2012; Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med (Berl). 90:625–35. https://doi.org/10.1007/s00109-011-0843-2. DOI: 10.1007/s00109-011-0843-2. PMID: 22187113.
Article22. Chen J, Singh K, Mukherjee BB, Sodek J. 1993; Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 13:113–23. https://doi.org/10.1016/s0934-8832(11)80070-3. DOI: 10.1016/S0934-8832(11)80070-3.
Article23. Prosecká E, Rampichová M, Vojtová L, Tvrdík D, Melčáková S, Juhasová J, et al. 2011; Optimized conditions for mesenchymal stem cells to differentiate into osteoblasts on a collagen/hydroxyapatite matrix. J Biomed Mater Res A. 99:307–15. https://doi.org/10.1002/jbm.a.33189. DOI: 10.1002/jbm.a.33189. PMID: 21858919.
Article24. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. 2007; Endocrine regulation of energy metabolism by the skeleton. Cell. 130:456–69. https://doi.org/10.1016/j.cell.2007.05.047. DOI: 10.1016/j.cell.2007.05.047. PMID: 17693256. PMCID: PMC2013746.
Article25. Blázquez-Medela AM, López-Novoa JM, Martínez-Salgado C. 2011; Osteoprotegerin and diabetes-associated pathologies. Curr Mol Med. 11:401–16. https://doi.org/10.2174/156652411795976565. DOI: 10.2174/156652411795976565. PMID: 21568931.
Article26. Marks SC Jr, Odgren PR. Bilezikian JP, Raisz LG, Rodan GA, editors. 2002. Chapter 1 - structure and development of the skeleton. Principles of bone biology. 2nd ed. Academic Press;New York (NY): p. 3–15. DOI: 10.1016/B978-012098652-1.50103-7. PMCID: PMC65695.27. Liu X, Wu H, Byrne M, Krane S, Jaenisch R. 1997; Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 94:1852–6. https://doi.org/10.1073/pnas.94.5.1852. DOI: 10.1073/pnas.94.5.1852. PMID: 9050868. PMCID: PMC20006.
Article28. Song DS, Park JC, Jung IH, Choi SH, Cho KS, Kim CK, et al. 2011; Enhanced adipogenic differentiation and reduced collagen synthesis induced by human periodontal ligament stem cells might underlie the negative effect of recombinant human bone morphogenetic protein-2 on periodontal regeneration. J Periodontal Res. 46:193–203. https://doi.org/10.1111/j.1600-0765.2010.01328.x. DOI: 10.1111/j.1600-0765.2010.01328.x. PMID: 21118417.
Article29. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. 2002; The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 108:17–29. https://doi.org/10.1016/s0092-8674(01)00622-5. DOI: 10.1016/S0092-8674(01)00622-5.
Article30. Shi S, Cheng X, Wang J, Zhang W, Peng L, Zhang Y. 2009; RhBMP-2 microspheres-loaded chitosan/collagen scaffold enhanced osseointegration: an experiment in dog. J Biomater Appl. 23:331–46. https://doi.org/10.1177/0885328208090013. DOI: 10.1177/0885328208090013. PMID: 18667455.
Article31. Chatzinikolaidou M, Lichtinger TK, Müller RT, Jennissen HP. 2010; Peri-implant reactivity and osteoinductive potential of immobilized rhBMP-2 on titanium carriers. Acta Biomater. 6:4405–21. https://doi.org/10.1016/j.actbio.2010.06.009. DOI: 10.1016/j.actbio.2010.06.009. PMID: 20558328.
Article32. Lan J, Wang ZF, Shi B, Xia HB, Cheng XR. 2007; The influence of recombinant human BMP-2 on bone-implant osseointegration: biomechanical testing and histomorphometric analysis. Int J Oral Maxillofac Surg. 36:345–9. https://doi.org/10.1016/j.ijom.2006.10.019. DOI: 10.1016/j.ijom.2006.10.019. PMID: 17300917.
Article33. Bae IH, Yun KD, Kim HS, Jeong BC, Lim HP, Park SW, et al. 2010; Anodic oxidized nanotubular titanium implants enhance bone morphogenetic protein-2 delivery. J Biomed Mater Res B Appl Biomater. 93:484–91. https://doi.org/10.1002/jbm.b.31606. DOI: 10.1002/jbm.b.31606. PMID: 20186826.
Article34. Shi J, Dong LL, He F, Zhao S, Yang GL. 2013; Osteoblast responses to thin nanohydroxyapatite coated on roughened titanium surfaces deposited by an electrochemical process. Oral Surg Oral Med Oral Pathol Oral Radiol. 116:e311–6. https://doi.org/10.1016/j.oooo.2012.02.021. DOI: 10.1016/j.oooo.2012.02.021. PMID: 22841429.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Periimplant Bone Regeneration in Hydroxyapatite Block Grafts with Mesenchymal Stem Cells and Bone Morphogenetic Protein-2
- A study on the effect of rotational speeds of the trephine mill on the temperature of surrounding bone during dental implantation procedure and osseointegration of implants
- Combined effects of rhBMP-2 and rhVEGF coated onto implants on osseointegration: pilot study
- Teh Effect of Hydroxyapatite Coating on the Mechanical Strengths and Histologic Profiles of Porous Titanium Implants in Dogs
- Novel analysis model for implant osseointegration using ectopic bone formation via the recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system in rats: a proof-of-concept study