Korean J Physiol Pharmacol.
2022 Jan;26(1):15-24. 10.4196/kjpp.2022.26.1.15.
Gallic acid-mitochondria targeting sequence-H3R9 induces mitochondria-targeted cytoprotection
- Affiliations
-
- 1Department of Physiology, Cardiovascular and Metabolic Disease Center, Smart Marine Therapeutic Center, Inje University College of Medicine, Busan 47392, Korea
- 2Division of Applied Medicine, Research Institute for Korea Medicine, School of Korean Medicine, Pusan National University, Yangsan 50612, Korea
- 3Department of Biology and Clinical Pharmacology, R&D Center, Samyang Biopharmaceuticals Corporation, Seongnam 13488, Korea
- 4Department of Internal Medicine, Sanggye Paik Hospital, Cardiovascular and Metabolic Disease Center, Inje University, Seoul 01757, Korea
- KMID: 2524507
- DOI: http://doi.org/10.4196/kjpp.2022.26.1.15
Abstract
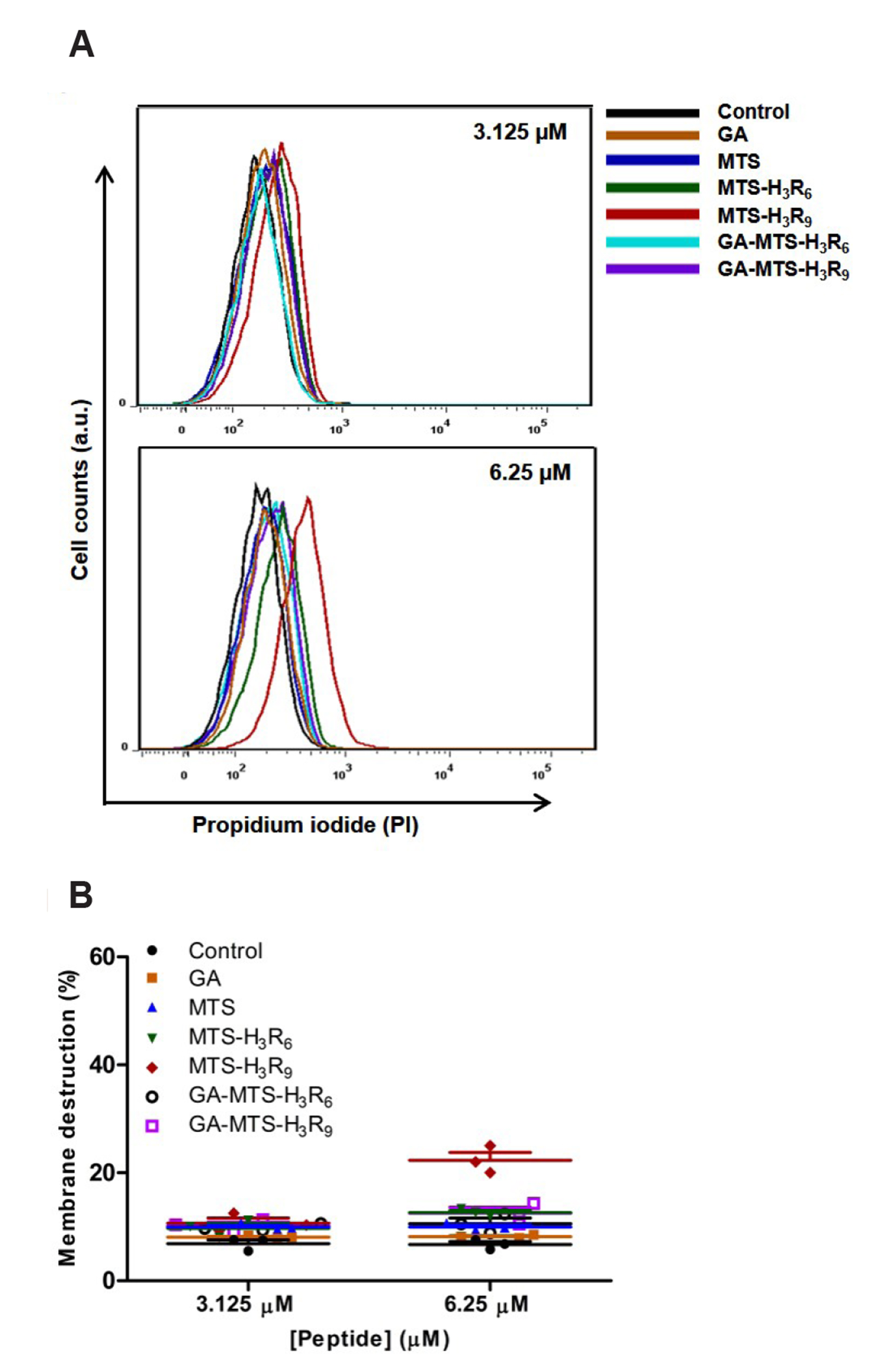
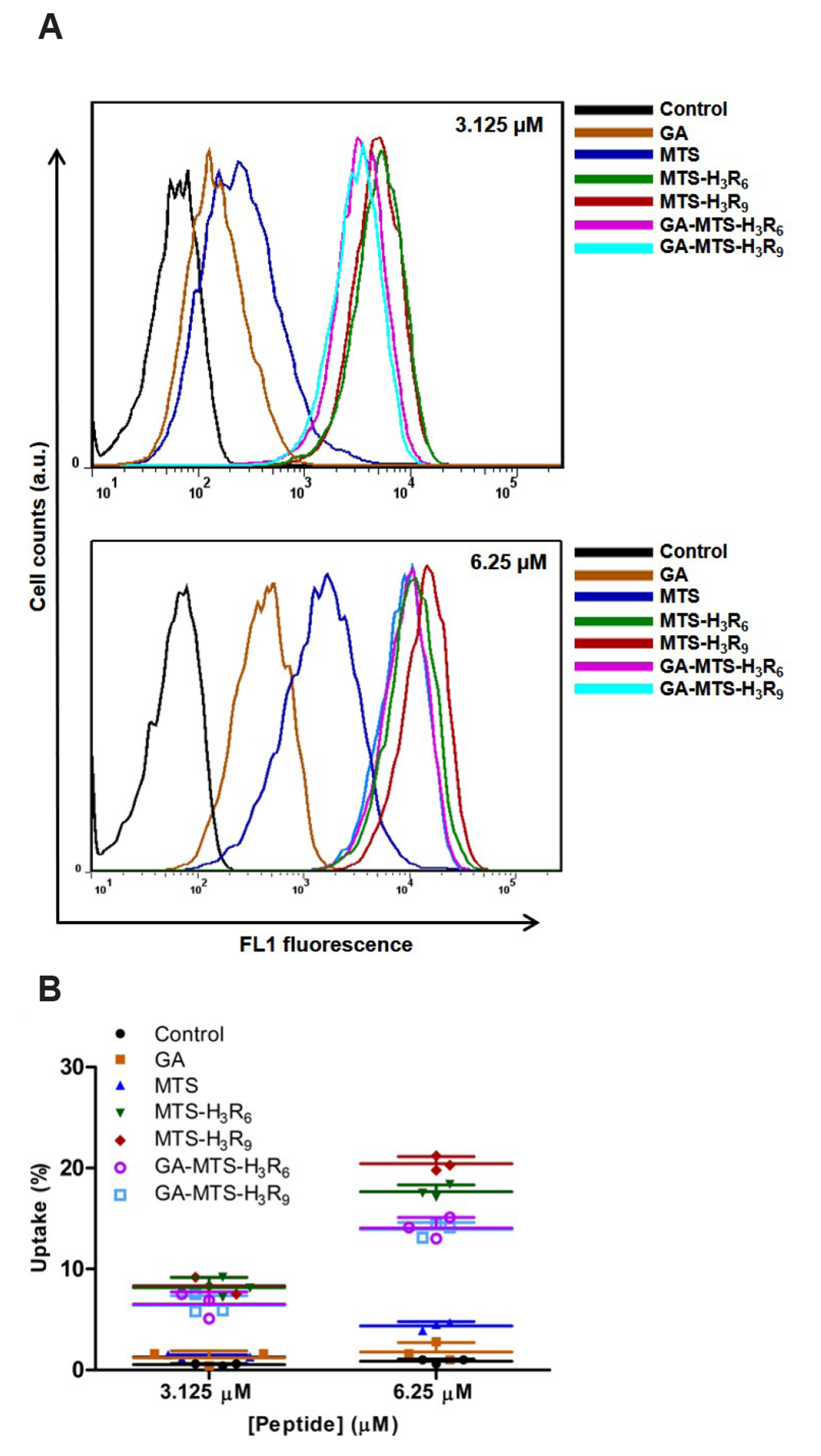
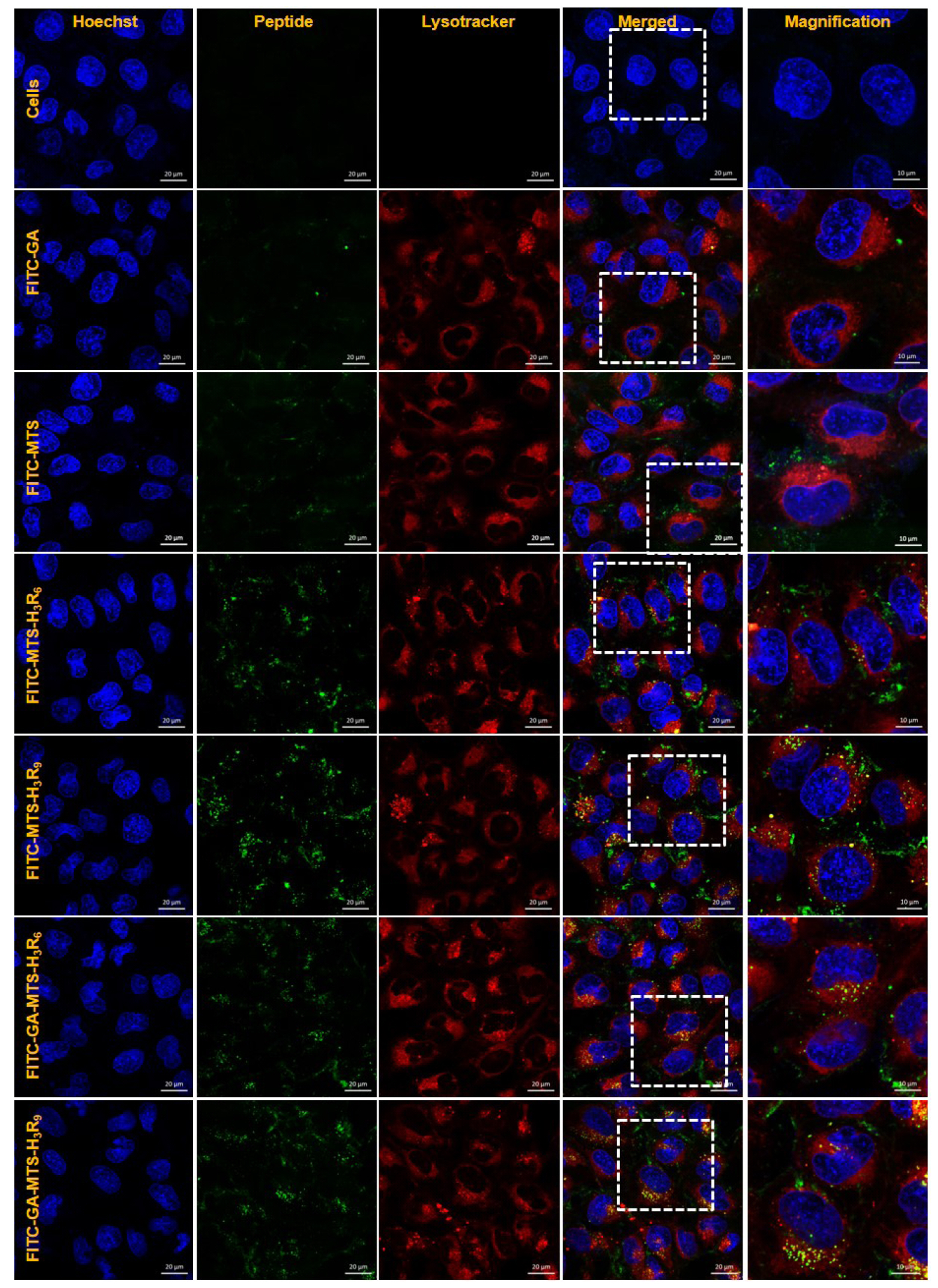
- The development of selective targeting of drug molecules towards the mitochondria is an important issue related to therapy efficacy. In this study, we report that gallic acid (GA)-mitochondria targeting sequence (MTS)-H3R9 exhibits a dual role as a mitochondria-targeting vehicle with antioxidant activity for disease therapy. In viability assays, GA-MTS-H3R9 showed a better rescue action compared to that of MTS-H3R9 . GA-MTS-H3R9 dramatically exhibited cell penetration and intercellular uptake compared to MTS and fit escape from lysosome release to the cytosol. We demonstrated the useful targeting of GA-MTS-H3R9 towards mitochondria in AC16 cells. Also, we observed that the antioxidant properties of mitochondrial-accrued GA-MTSH3R9 alleviated cell damage by reactive oxygen species production and disrupted mitochondrial membrane potential. GA-MTS-H3R9 showed a very increased cytoprotective effect against anticancer activity compared to that of MTS-H3R9 . We showed that GA-MTS-H3R9 can act as a vehicle for mitochondria-targeting and as a reagent for therapeutic applications intended for cardiovascular disease treatment.
Keyword
Figure
Reference
-
1. Wolfram JA, Donahue JK. 2013; Gene therapy to treat cardiovascular disease. J Am Heart Assoc. 2:e000119. DOI: 10.1161/JAHA.113.000119. PMID: 23963752. PMCID: PMC3828796.
Article2. Leo CH, Jelinic M, Ng HH, Parry LJ, Tare M. 2019; Recent developments in relaxin mimetics as therapeutics for cardiovascular diseases. Curr Opin Pharmacol. 45:42–48. DOI: 10.1016/j.coph.2019.04.001. PMID: 31048209.
Article3. Rossignol P, Hernandez AF, Solomon SD, Zannad F. 2019; Heart failure drug treatment. Lancet. 393:1034–1044. DOI: 10.1016/S0140-6736(18)31808-7. PMID: 30860029.
Article4. Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS. 2011; Nanoparticles targeting the infarcted heart. Nano Lett. 11:4411–4414. DOI: 10.1021/nl2025882. PMID: 21899318. PMCID: PMC3192253.
Article5. Tan KX, Pan S, Jeevanandam J, Danquah MK. 2019; Cardiovascular therapies utilizing targeted delivery of nanomedicines and aptamers. Int J Pharm. 558:413–425. DOI: 10.1016/j.ijpharm.2019.01.023. PMID: 30660748.
Article6. Mahapatro A, Singh DK. 2011; Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 9:55. DOI: 10.1186/1477-3155-9-55. PMID: 22123084. PMCID: PMC3238292.
Article7. Sanna V, Pala N, Sechi M. 2014; Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 9:467–483. DOI: 10.2147/IJN.S36654. PMID: 24531078. PMCID: PMC3896284.8. Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C. 2019; Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater. 10:4. DOI: 10.3390/jfb10010004. PMID: 30626094. PMCID: PMC6463038.
Article9. Cosentino K, García-Sáez AJ. 2014; Mitochondrial alterations in apoptosis. Chem Phys Lipids. 181:62–75. DOI: 10.1016/j.chemphyslip.2014.04.001. PMID: 24732580.
Article10. Rin Jean S, Tulumello DV, Wisnovsky SP, Lei EK, Pereira MP, Kelley SO. 2014; Molecular vehicles for mitochondrial chemical biology and drug delivery. ACS Chem Biol. 9:323–333. DOI: 10.1021/cb400821p. PMID: 24410267.
Article11. Weissig V, Boddapati SV, Jabr L, D'Souza GG. 2007; Mitochondria-specific nanotechnology. Nanomedicine (Lond). 2:275–285. DOI: 10.2217/17435889.2.3.275. PMID: 17716174.
Article12. Lin R, Zhang P, Cheetham AG, Walston J, Abadir P, Cui H. 2015; Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug Chem. 26:71–77. DOI: 10.1021/bc500408p. PMID: 25547808. PMCID: PMC4306504.
Article13. Klimpel A, Neundorf I. 2018; Bifunctional peptide hybrids targeting the matrix of mitochondria. J Control Release. 291:147–156. DOI: 10.1016/j.jconrel.2018.10.029. PMID: 30367921.
Article14. von Heijne G. 1986; Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 5:1335–1342. DOI: 10.1002/j.1460-2075.1986.tb04364.x. PMID: 3015599. PMCID: PMC1166945.
Article15. Yu GS, Han J, Ko KS, Choi JS. 2014; Cationic oligopeptide-conjugated mitochondria targeting sequence as a novel carrier system for mitochondria. Macromol Res. 22:42–46. DOI: 10.1007/s13233-014-2003-3.
Article16. Schmidt N, Mishra A, Lai GH, Wong GC. 2010; Arginine-rich cell-penetrating peptides. FEBS Lett. 584:1806–1813. DOI: 10.1016/j.febslet.2009.11.046. PMID: 19925791.
Article17. Bae Y, Joo C, Kim GY, Ko KS, Huh KM, Han J, Choi JS. 2019; Cationic oligopeptide-functionalized mitochondria targeting sequence show mitochondria targeting and anticancer activity. Macromol Res. 27:1071–1080. DOI: 10.1007/s13233-019-7153-x.
Article18. Xie M, Hu B, Wang Y, Zeng X. 2014; Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J Agric Food Chem. 62:9128–9136. DOI: 10.1021/jf503207s. PMID: 25198516.
Article19. Perron NR, Brumaghim JL. 2009; A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 53:75–100. DOI: 10.1007/s12013-009-9043-x. PMID: 19184542.
Article20. Bae Y, Green ES, Kim GY, Song SJ, Mun JY, Lee S, Park JI, Park JS, Ko KS, Han J, Choi JS. 2016; Dipeptide-functionalized polyamidoamine dendrimer-mediated apoptin gene delivery facilitates apoptosis of human primary glioma cells. Int J Pharm. 515:186–200. DOI: 10.1016/j.ijpharm.2016.09.083. PMID: 27732896.
Article21. Bae Y, Jung MK, Lee S, Song SJ, Mun JY, Green ES, Han J, Ko KS, Choi JS. 2018; Dequalinium-based functional nanosomes show increased mitochondria targeting and anticancer effect. Eur J Pharm Biopharm. 124:104–115. DOI: 10.1016/j.ejpb.2017.12.013. PMID: 29305141.
Article22. AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. 2009; Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 3:279–290. DOI: 10.1021/nn800596w. PMID: 19236062.
Article23. Holder AL, Goth-Goldstein R, Lucas D, Koshland CP. 2012; Particle-induced artifacts in the MTT and LDH viability assays. Chem Res Toxicol. 25:1885–1892. DOI: 10.1021/tx3001708. PMID: 22799765. PMCID: PMC3446248.
Article24. Yang Y, Xiang Y, Xu M. 2015; From red to green: the propidium iodide-permeable membrane of Shewanella decolorationis S12 is repairable. Sci Rep. 5:18583. DOI: 10.1038/srep18583. PMID: 26687136. PMCID: PMC4685271.
Article25. Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu S, Li J, Pang X, Shi H, Liang H. 2013; Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 1833:2996–3005. DOI: 10.1016/j.bbamcr.2013.08.003. PMID: 23954443.
Article26. Soumya RS, Vineetha VP, Salin Raj P, Raghu KG. 2014; Beneficial properties of selenium incorporated guar gum nanoparticles against ischemia/reperfusion in cardiomyoblasts (H9c2). Metallomics. 6:2134–2147. DOI: 10.1039/C4MT00241E. PMID: 25307064.
Article27. He H, Li DW, Yang LY, Fu L, Zhu XJ, Wong WK, Jiang FL, Liu Y. 2015; A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells. Sci Rep. 5:13543. DOI: 10.1038/srep13543. PMID: 26337336. PMCID: PMC4559806.
Article28. Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. 2012; JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 3:e430. DOI: 10.1038/cddis.2012.171. PMID: 23171850. PMCID: PMC3542606.
Article29. Sancho P, Galeano E, Nieto E, Delgado MD, García-Pérez AI. 2007; Dequalinium induces cell death in human leukemia cells by early mitochondrial alterations which enhance ROS production. Leuk Res. 31:969–978. DOI: 10.1016/j.leukres.2006.11.018. PMID: 17250890.
Article30. Madani F, Lindberg S, Langel U, Futaki S, Gräslund A. 2011; Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011:414729. DOI: 10.1155/2011/414729. PMID: 21687343. PMCID: PMC3103903.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Interplay between Autophagy and Aging
- Understanding the Role of Heat Shock Protein Isoforms in Male Fertility, Aging and Apoptosis
- Ultrastructural Studies on Mitochondria of Preimplantaion Rabbit Embryos
- Myopathy, Drugs, and Mitochondria
- The Formation of Giant Mitochondria in the Liver Cells Induced by Hydrazine