Korean J Transplant.
2021 Dec;35(4):253-256. 10.4285/kjt.21.0025.
Acute T cell-mediated rejection after administration of the BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient: a case report
- Affiliations
-
- 1Division of Kidney and Pancreas Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2523675
- DOI: http://doi.org/10.4285/kjt.21.0025
Abstract
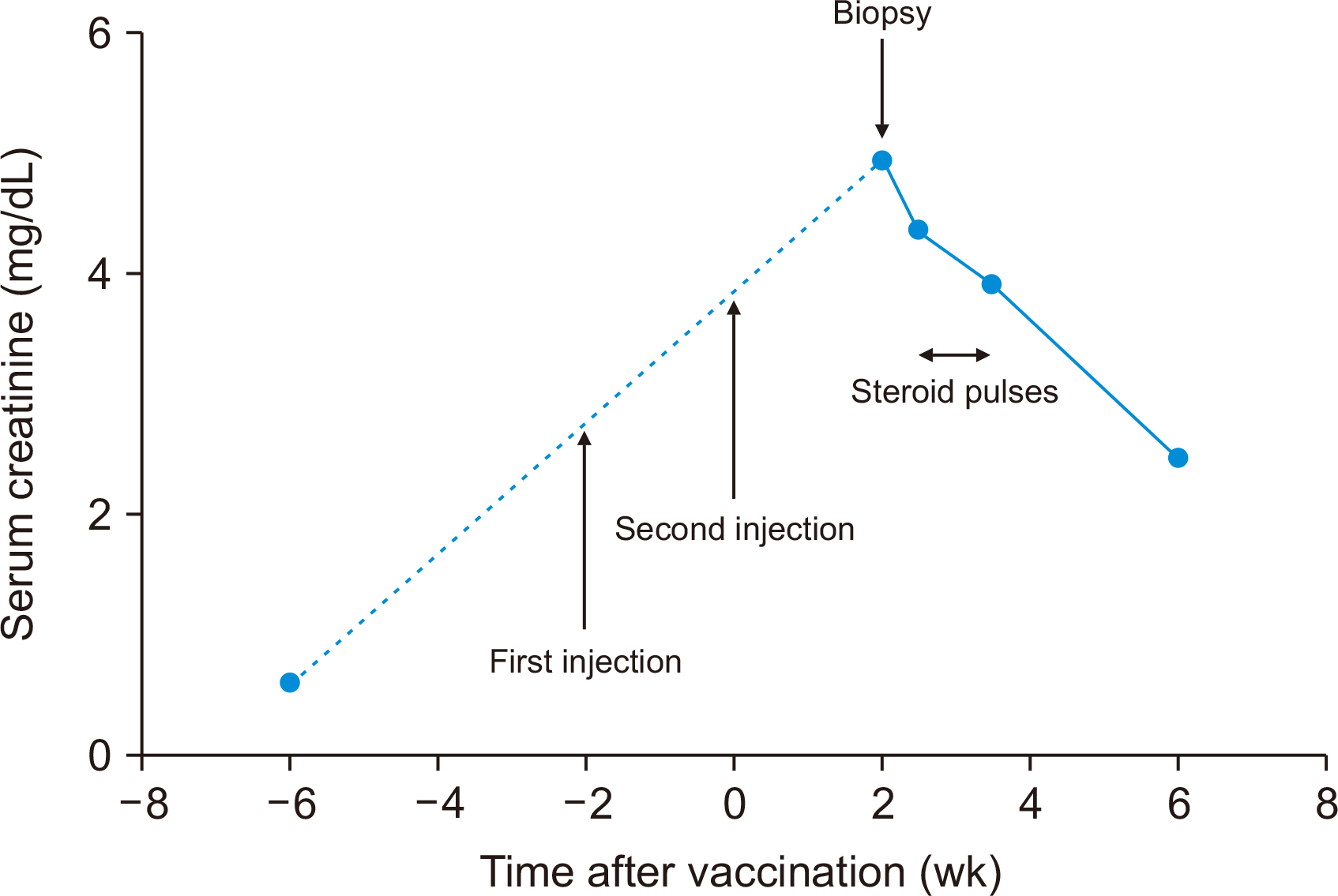
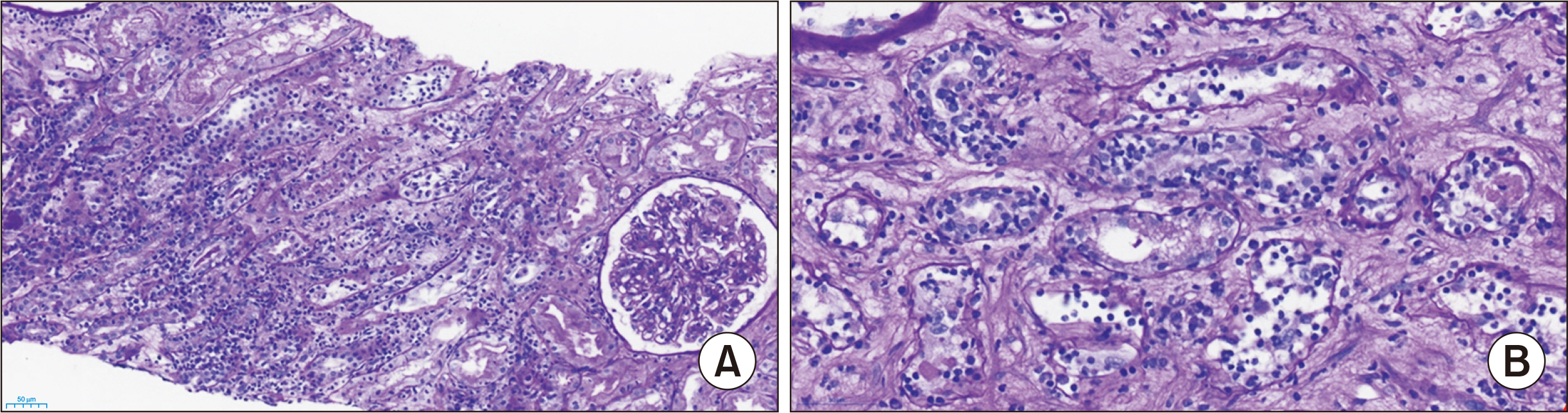
- The impact of the coronavirus disease 2019 (COVID-19) vaccination on humoral and cellular immunity in transplant recipients remains unknown. We report the case of a 78-year-old kidney transplant recipient who experienced acute T cell-mediated rejection after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech). She had no history of acute rejection throughout the 13 years after deceased donor kidney transplantation. Fifteen days after receiving the second dose of the BNT162b2 vaccine, the recipient visited our center with a mild headache and fever. Her serum creatinine level had increased from 0.61 to 4.95 mg/dL. Kidney allograft biopsy indicated acute T cell-mediated rejection (grade IB) with no pathologic evidence of antibody-mediated rejection. Anti-severe acute respiratory syndrome coronavirus 2 spike-immunoglobulin G and -immunoglobulin M measurements were weak positive and negative, respectively. Careful monitoring of kidney allograft function is vital for transplant recipients undergoing COVID-19 vaccination.
Figure
Reference
-
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. 2020; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 55:105924. DOI: 10.1016/j.ijantimicag.2020.105924. PMID: 32081636. PMCID: PMC7127800.
Article2. Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. 2020; Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 98:1540–8. DOI: 10.1016/j.kint.2020.09.006. PMID: 32979369. PMCID: PMC7560263.
Article3. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. 2021; Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 73:e4090–9. DOI: 10.1093/cid/ciaa1097. PMID: 32766815. PMCID: PMC7454362.4. Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, et al. 2020; SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 20:3008–18. DOI: 10.1111/ajt.16247. PMID: 32780493. PMCID: PMC7436919.
Article5. Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, et al. 2020; Banff 2019 meeting report: molecular diagnostics in solid organ transplantation-Consensus for the Banff Human Organ Transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant. 20:2305–17. DOI: 10.1111/ajt.16059. PMID: 32428337. PMCID: PMC7496585.
Article6. American Society of Transplantation. 2021. COVID-19: vaccine FAQ sheet [Internet]. Mt. Laurel, NJ;Available from: https://www.myast.org/covid-19-vaccine-faq-sheet. cited 2021 Jan 19.7. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. 2020; Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382:1708–20. DOI: 10.1056/NEJMoa2002032. PMID: 32109013. PMCID: PMC7092819.
Article8. BioNTech SE. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. Bethesda, MA: ClinicalTrials.gov;2020.9. ModernaTX. A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19. Bethesda, MA: ClinicalTrials.gov;2020.10. Del Bello A, Marion O, Delas A, Congy-Jolivet N, Colombat M, Kamar N. 2021; Acute rejection after anti-SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 100:238–9. DOI: 10.1016/j.kint.2021.04.025. PMID: 33932459. PMCID: PMC8080493.
Article11. Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, et al. 2021; Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 105:2170–4. DOI: 10.1097/TP.0000000000003780. PMID: 33859151. PMCID: PMC8487696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute T cell-mediated rejection after administration of the BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient without a history of acute rejection for 13 years: a case report
- Case Reports of Acute Transverse Myelitis Associated With mRNA Vaccine for COVID-19
- Comparison of kidney transplant outcomes before and after COVID-19 pandemic: a single-institution experience
- Adult-onset Still’s Disease after BNT162b2 mRNA COVID-19 Vaccine
- mRNA COVID-19 Vaccine-Associated Subserosal Eosinophilic Gastroenteritis: A Case Report