J Korean Med Sci.
2022 Aug;37(30):e233. 10.3346/jkms.2022.37.e233.
mRNA COVID-19 Vaccine-Associated Subserosal Eosinophilic Gastroenteritis: A Case Report
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- KMID: 2532227
- DOI: http://doi.org/10.3346/jkms.2022.37.e233
Abstract
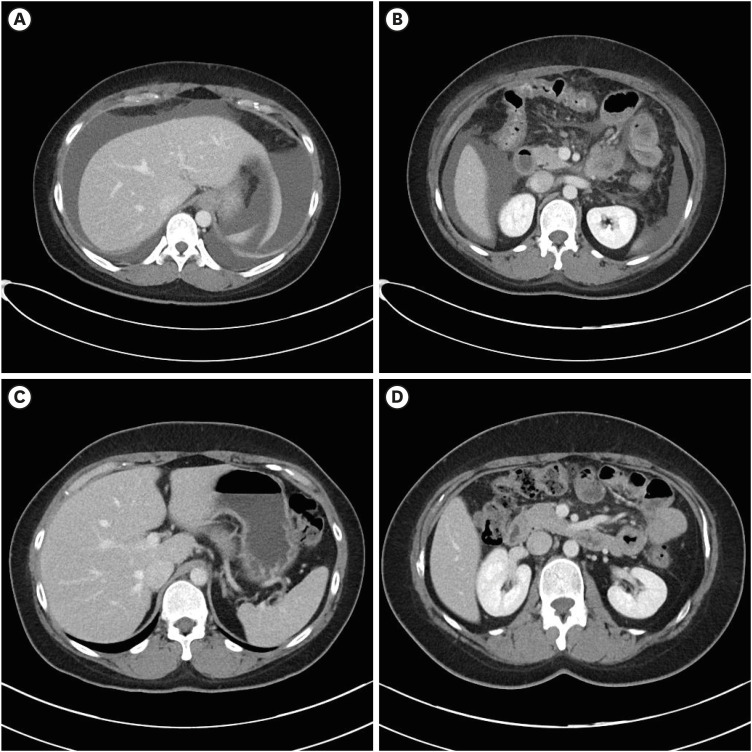
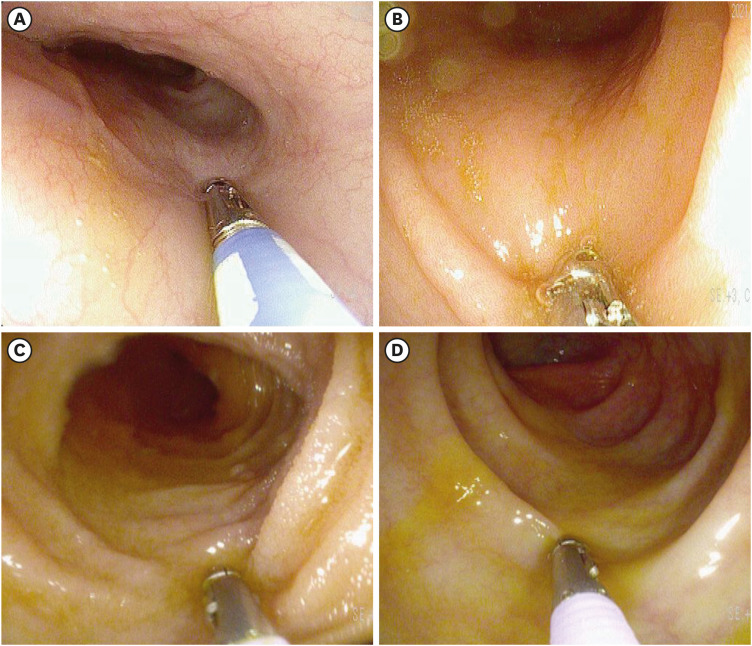
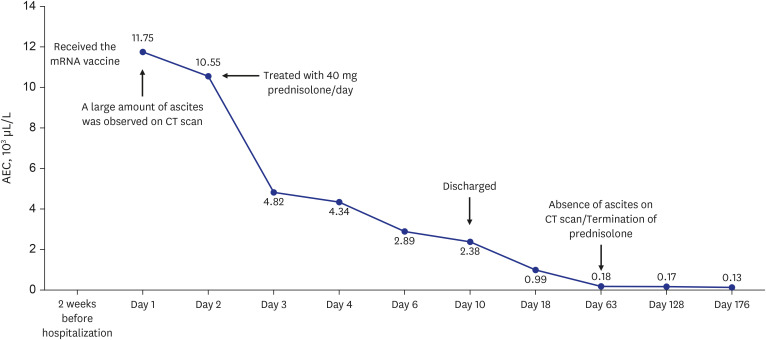
- The World Health Organization declared coronavirus disease 2019 (COVID-19) a global pandemic in March 2020. Several vaccines have been developed to overcome the COVID-19 pandemic, and messenger RNA vaccines, commonly known as mRNA vaccines, were the first COVID-19 vaccines to be authorized in Korea. With the worldwide increase in vaccinations, reports of adverse reactions are increasing. However, to the best of our knowledge, there have been no reports of eosinophilic gastroenteritis (EGE) following mRNA vaccination. Here, we present the first case of EGE in a patient who received a second dose of the mRNA vaccine, BNT162b2 (Pfizer-BioNTech). A previously healthy 34-year-old woman presented to the emergency department with generalized abdominal pain for the preceding 2 weeks. She had received a second dose of the mRNA COVID-19 vaccine 2 weeks prior. Subserosal EGE was diagnosed, oral prednisolone was administered, and she recovered completely.
Keyword
Figure
Reference
-
1. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020; 13(5):667–673. PMID: 32340833.
Article2. Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021; 170:1–25. PMID: 33359141.
Article3. Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990; 31(1):54–58. PMID: 2318432.
Article4. Tian XQ, Chen X, Chen SL. Eosinophilic gastroenteritis with abdominal pain and ascites: A case report. World J Clin Cases. 2021; 9(17):4238–4243. PMID: 34141786.
Article5. Bischoff SC, Ulmer FA. Eosinophils and allergic diseases of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008; 22(3):455–479. PMID: 18492566.
Article6. Yun MY, Cho YU, Park IS, Choi SK, Kim SJ, Shin SH, et al. Eosinophilic gastroenteritis presenting as small bowel obstruction: a case report and review of the literature. World J Gastroenterol. 2007; 13(11):1758–1760. PMID: 17461485.
Article7. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002; 168(5):2464–2469. PMID: 11859139.
Article8. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003; 125(5):1419–1427. PMID: 14598258.
Article9. Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014; 43(2):257–268. PMID: 24813514.
Article10. Zhang M, Li Y. Eosinophilic gastroenteritis: a state-of-the-art review. J Gastroenterol Hepatol. 2017; 32(1):64–72.
Article11. Michet CJ Jr, Rakela J, Luthra HS. Auranofin-associated colitis and eosinophilia. Mayo Clin Proc. 1987; 62(2):142–144. PMID: 3807438.
Article12. Riedel RR, Schmitt A, de Jonge JP, Hartmann A. Gastrointestinal type 1 hypersensitivity to azathioprine. Klin Wochenschr. 1990; 68(1):50–52. PMID: 2308269.
Article13. Ravi S, Holubka J, Veneri R, Youn K, Khatib R. Clofazimine-induced eosinophilic gastroenteritis in AIDS. Am J Gastroenterol. 1993; 88(4):612–613.14. Lee JY, Medellin MV, Tumpkin C. Allergic reaction to gemfibrozil manifesting as eosinophilic gastroenteritis. South Med J. 2000; 93(8):807–808. PMID: 10963515.
Article15. Barak N, Hart J, Sitrin MD. Enalapril-induced eosinophilic gastroenteritis. J Clin Gastroenterol. 2001; 33(2):157–158. PMID: 11468446.
Article16. Shakeer VK, Devi SR, Chettupuzha AP, Mustafa CP, Sandesh K, Kumar SK, et al. Carbamazepine-induced eosinophilic enteritis. Indian J Gastroenterol. 2002; 21(3):114–115. PMID: 12118924.17. Tomiyasu H, Komori T, Ishida Y, Otsuka A, Kabashima K. Eosinophilic gastroenteritis in a melanoma patient treated with nivolumab. J Dermatol. 2021; 48(10):E486–E487. PMID: 34151453.
Article18. Wienand B, Sanner B, Liersch M. Eosinophilic gastroenteritis as an allergic reaction to a trimethoprim-sulfonamide preparation. Dtsch Med Wochenschr. 1991; 116(10):371–374. PMID: 2001640.
Article19. de Las Vecillas L, López J, Morchón E, Rodriguez F, Drake M, Martino M. Viral-like reaction or hypersensitivity? Erythema multiforme minor reaction and moderate eosinophilia after the Pfizer-BioNTech BNT162b2 (mRNA-Based) SARS-CoV-2 vaccine. J Investig Allergol Clin Immunol. 2021; 32(1):77–78.
Article20. Ishizuka K, Katayama K, Kaji Y, Tawara J, Ohira Y. Non-episodic angioedema with eosinophilia after BNT162b2 mRNA COVID-19 vaccination. QJM. 2021; 114(10):745–746. PMID: 34546346.
Article21. de Montjoye L, Marot L, Baeck M. Eosinophilic cellulitis after BNT162b2 mRNA Covid-19 vaccine. J Eur Acad Dermatol Venereol. 2022; 36(1):e26–e28. PMID: 34547138.
Article22. O’Connor T, O’Callaghan-Maher M, Ryan P, Gibson G. Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID-19 vaccine. JAAD Case Rep. 2022; 20:14–16. PMID: 34931172.
Article